Year: 2003 Vol. 69 Ed. 3 - (5º)
Artigo Original
Pages: 318 to 325
Nasal polyposys: a chronic inflammatory progressive disease?
Author(s):
Bruno Beltrão de Souza[1],
Marcelo Fenile Serra1,
João Vicente Dorgam1,
Sabrina Maria de Castro Sarreta1,
Valder Rodrigues de Melo[2],
Wilma T. Anselmo-Lima[3]
Keywords: polyposis, chronic inflammatory disease, ultrastructural alterations.
Abstract:
Introduction: Nasosinusal polyposis is a degenerative disease of the mucosa with the formation of multiple polypoid structures in the nasal cavities and facial sinuses whose physiopathological mechanisms are not well understood. Objectives, Material and Methods: In order to contribute to the understanding of the factors involved in the pathogenesis of nasal polyps we decided to study the histology and ultrastructure of the polyps in 17 patients with polyposis. The patients were divided into two groups: allergic (5 patients) and non-allergic (12 patients). Results: A normal respiratory mucosa covering the surface of the epithelium was detected in eight cases, squamous metaplasia in four, different epithelial types in three, atypical respiratory epithelium in one, and absence of mucosa in one. We observed 14 cases of polyps of the fibro-inflammatory type, one case of seromucinous gland hyperplasia, and two cases of fibrotic polyps. Conclusions: The patients presented rates of positive skin test equal to or higher than those of the general population. However, there were no histological or ultrastructural differences between the polyps of allergic and non-allergic patients, suggesting that allergy is a contributing but not a causal factor in the physiopathology of nasosinusal polyposis.
![]()
INTRODUCTION
Nasosinusal Polyposis (NP) is a chronic inflammatory disease of nasal respiratory mucosa and paranasal sinuses. Its clinical symptoms are typically bilateral polypoid formation typically leading to nasal obstruction, rhinorrhea, hyposmia or anosmia and recurrent rhinosinusitis. According to Caplin et al, 1971(1), it affects 0.5 to 4% of the population, and its pathogenesis, allergy relation and treatment of choice are still controversial.
The chronic inflammatory process in the submucosa seems to be a common symptom in all NP cases, and could be evidenced in several papers in the literature. According to Norlander et al(2), 1999, there is considerable increment of inflammatory mediators in the polypoid submucosa and surrounding mucosa. Cytokine-combined effects and growth factors produced by T lymphocytes, fibroblasts, epithelial cells, and blood stream cells, primarily eosinophils, could be responsible for several phases of the polyp formation process.
Pathogenesis of Nasosinusal polyposis (NP) still has several aspects to be understood. Chronic inflammatory process is one of the major symptoms involved with it, however not all inflammatory diseases of the mucosa occur with Nasosinusal Polyposis, which is the case of chronic rhinosinusitis. Likewise, decrease of airflow due to anatomic blockage with subsequent reduction of tissue O2 occurred in most of the cases, but it is not a factor strictly required in the pathogenesis of the disease.
Currently, the literature presents some tendency to consider NP as an inflammatory multi-factorial disease. Local factors such as bacterial infection or structural changes such as septum deviation and anatomical variations of medial meatus lead to local inflammatory response, which causes the onset of mucosa ulceration with subsequent collapse of the submucosa, which triggers reepithelialization, and glandular proliferation. Fibroblasts and epithelial cells act by producing cytokines, chemotactic factors and other mediators that will sustain the inflammatory process. As a result of all those symptoms, ion absorption in stroma will be changed, as demonstrated in some studies of the same author, increasing the edema and leading to polypoid growth. Genetic factors also play a role in this pathogenesis.
The objective of this study is to analyze morphological structural changes in NP using optical and electron microscopy focusing mainly on inflammatory infiltrate and the likely role of allergy in this pathophysiology.
MATERIAL AND METHODS
Seventeen patients with nasosinusal polyposis were studied in the Laboratory of Rhinosinusology. Patients were aged from 9 to 77 years with mean age of 42.6 years. Nine patients were male and eight were female.
Inclusion Criteria: This study included patients with bilateral nasosinusal polyposis that did not respond to therapy after 6 months of conventional clinical treatment based on topic and systemic use of steroids and wide spectrum antibiotics, and were then submitted to surgical treatment. Specimens were collected through endoscope surgical procedure.
Exclusion criteria: patients with associated diseases, primarily cystic fibrosis, cilia dyskinesis and Young and Churg-Strauss Syndromes were excluded.
The study was approved by the Ethics Committee on Research of HC-FMRP-USP in compliance with Process 1930/97.
Patients were separated in two groups:
- Allergic Patients (05 patients)
Patients with clinical picture compatible with allergic rhinitis (nasal obstruction, sneezes, rhinorrhea and pruritus); positive hypersensitiveness skin test.
- Non-allergic Patients (12 patients)
Absence of allergic rhinitis symptoms: negative hypersensitiveness skin test.
Material Preparation for Histological Examination and Ultra structural Optical Microscopy (OM)
Polyps were classified according to their histology in four types: eosinophilic, fibrous-inflammatory, hyperplastic polyp with seromucinous glands and polyp with atypical stroma. Polyp collection was performed during the surgery ands then stained with hematoxyllin and eosin.
Electron Transmission Microscopy
Polyp fragments collected (0.3 mm diameter each) were washed in saline solution (S.F. 0.9%), were stained with glutaraldehyde (3%) for 2 hours at 4 degrees C. After that, stained fragments were washed in phosphate buffer (0.1M). Next, they were post-stained with Osmium Tetra oxide at 1% for 2 hours at 4 degrees C, washed three times in phosphate buffer (0.1M), and then were dehydrated with incremental series of acetone as follows: 30, 50, 70, 90, 95 and 100%. Subsequently, they were included in Araldite Resin for 72 hours at 60 degrees C, and semi-fine and ultra fine slices were performed with glass knife and diamond (respectively). Semi-fine slices were dyed with Toluidine Blue and photographed under Zeiss microscope. Ultra fine slices (600 A) were collected in copper mesh (200 mesh); contrasted with Uranyl Acetate (4%); washed 4 times with water and Lead Citrate (0.3%) for 15 minutes; washed 4 times with MilliQ water. Slices were observed in a Phillips 208 electronic transmission microscope running at 80kv. Specimens were processed, observed and analyzed in the Laboratory of Electronic Microscopy of the Department of Morphology, Medical School of Ribeirão Preto -USP.
RESULTS
Patient Group Characterization
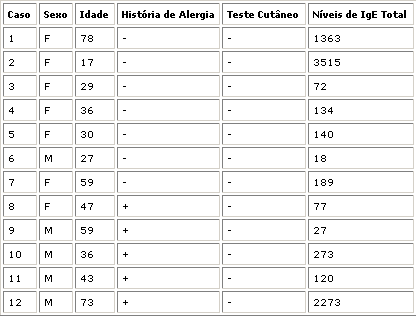
In an attempt to characterize 2 groups of allergic and non-allergic patients we decided to evaluate the patients by clinical history, skin test and total IgE values. Five patients were selected to form group 1 (allergic patients). These patients had a history of allergy and positive skin test. The other patients, although some had history of allergy and total IgE values above normal range, had negative skin tests and were then considered non-allergic (Tables I and II).
1. Histological and Ultra structural Study
Patients had several polyp samples exhaustively analyzed by optical and electronic transmission microscopy to characterize the type of mucosa epithelium. In group 1, we found two cases of normal respiratory epithelium, one patient with atypical respiratory epithelium, one metaplastic patient and one patient with absence of mucosa (Table III).
In Group 2, normal respiratory epithelium was found in 6 patients, three patients had different types of epithelium, and the last three patients presented metaplastic epithelium (Table IV).
The submucosa of patients in both groups was studied according to the classification proposed by Davidsson et al. in 1993(4).
In group 1 (allergic) three patients had fibrous-inflammatory polyps and two had fibroid polyps and were not found in the classification of Davidsson et al. (Table V).
In group 2 (non-allergic) we found one patient with seromucinous glands and the other 11 patients had fibrous-inflammatory polyps. Eosinophilic polyps and atypical stroma were not found or identified (Table VI).
Therefore, normal respiratory mucosa lining the polyp surface was found in 8 patients, or pseudostratified ciliate epithelium with goblet and cilia cells with normal morphology (Figure 1). The magnification described in the pictures represents the original one. Atypical respiratory epithelium was found in one case: significant decrease in the number of ciliate cells and cilia (Fig. 2). Four patients presented metaplastic epithelium with flat squamous cell and total absence of cilia (Fig. 3). Metaplastic squamous cells and atypical epithelium sites: mucosa with decreased count of ciliate cells with some cases of total absence of cilia (Fig. 4). One patient had absence of mucosa (Fig. 5).
All patients had their submucosa thoroughly studied by optical and electron transmission microscopy. Fourteen patients had fibrous-inflammatory polyp characterized by intense inflammatory infiltrate with mainly lymphocytes, plasmocytes, and neutrophils, massive fibrosis and fine collagen fibers (Fig. 6 and 7), one patient with seromucinous gland hyperplasia (Fig. 8) and two cases of highly fibroid submucosa (fibroid polyp - Fig. 9).Table I - Group 1: patients considered allergic.Table II - Group 2: patients considered non-allergic.
Table III- Types of epithelium found in patients in group 1.
Types of epithelium / Number of Cases
Normal respiratory epithelium - 2
Atypical respiratory epithelium - 1
Metaplastic epithelium - 1
Absence of mucosa - 1
Table IV - Types of epithelium found in patients in group 2.
Types of epithelium / Number of Cases
Normal respiratory epithelium - 6
Different types of epithelium - 3
Metaplastic epithelium - 3
Table V - Types of polyps found in patients in group 1.
Types of polyps / Number of Cases
Fibrous-inflammatory - 3
Fibrotic - 2
Table VI - Types of polyps found in patients in group 2.
Types of polyps / Number of Cases
Fibrous-inflammatory - 11
Seromucinous gland hyperplasia - 1
DISCUSSION
Although NP has been known since ancient times and has relatively high prevalence among all populations, its pathophysiology and effective treatment remains a challenge. The events that trigger polyp formation are not fully explained, but some progress may already be observed in this field.
Age and Gender
The mean age in the two groups was 42.6 years. There were no differences between mean age of the allergic group (41.6 years) and the non-allergic group (44 years). This mean age above 30 years was also reported by other authors (5,6,7), confirming that this pathology is fairly rare in children and teenagers.
Nine out of the seventeen patients studied were male (53.3%) and eight were female (46.6%), statistically compatible with the literature (5,6,7). Male patients were predominant in group 1 whereas in Group 2 the gender ratio was the same.
Skin Tests
Eight out of the seventeen patients with polyposis had positive skin test, a high rate (66%) comparable only to the study of WONG et al. (1992)(8) in which the control group with patients for surgery with no polyposis had a percentage of positive skin test that was even higher (74%). DRAKE LEE et al. (1984)(9) had a rate of 44% of patients with polyposis with positive skin test. Other researchers concluded that the rate of patients with positive skin test with Polyposis were similar to those of the population in general (approximately 30%)(5,6,7).
GERGEN et al. (1991)(10) also tested 16,204 patients to check the atopy rate in patients of general population and it was 20%. Such finding was also observed by KEITH et al. (1995)(11) that described that NP patients are more atopic than the population in general and less atopic than patients referred to allergy physicians, therefore supporting that allergy is a contributing factor for NP, which in our opinion is a coherent conclusion.
Optical Microscopy
DAVIDSSON et al. (1993)(4), developed the only classification we found in the literature studying polyps. The results of our study were totally different: total predominance of fibrous-inflammatory polyps, which could be explained by our small sample or by geographic and/or social variation of the population studied, or mainly by the fact that patients in our study presented advanced polyposis ranging from degrees IV and V of STAMM (1995)(12).
In terms of histological types, we found dense fibrosis, which we named fibrotic (2) cases. It is either a new type or a variation of the same polyp in relation to its structure due to a variation, which in our opinion is more likely to occur; if the histological section had been made at another location we probably would have been able to see the four types above mentioned.
No significant differences were found in microscopy concerning allergic and non-allergic groups. Such data is compatible with RUHNO et al. (1990)(13) that did not find any differences in mast cell number between allergic and non-allergic patients with NP. HAMILOS et al. (1995)(14) showed the absence of differences related to eosinophilia in NP allergic and non-allergic patients, and PARK et al. (1998)15) showed by histochemical analysis of cell infiltrate of nasal polyps that there were no differences between the allergic and non-allergic groups.
Electronic Transmission Microscopy
The majority of our NP samples presented cylindrical ciliate pseudostratified epithelium (47%), followed by metaplastic stratified pavement epithelium (23%). These data were compatible with studies of MYGIND et al. (1974)(16) and LARSEN & TOS (1990)(17). Two patients had intense secretion activity with large amount of mitochondria and well developed Golgi apparatus also found by CAUNA et al. (1972)(18) , but not observed by MYGIND et al. (1973 and 1974)(16,19).
In reference to group 1 (allergic) and 2 (non-allergic), no differences were found between them and it was also reported by MYGIND et al. (1974) and other authors (16,19).
MYGIND & BRETLAU (1973 and 1974)(16,19) did not find any significant ultra structural differences between the normal epithelium of the inferior nasal turbinate and the epithelium that covered the polyps. Some morphological details, however, were described by CAUNA (1972)(18), such as intercellular spaces distended due to edema, citoplasmatic vacuolation, and glycogen granules build up in some epithelial cells, hyperplastic goblet cells, in addition to the presence of inflammatory cells, specially neutrophils, mast cells and eosinophils.
The inflammatory site
According to NORLANDER et al. (1996 and 1999)(2,20) many current research studies about polyps have mentioned the accumulation of eosinophils in pathological tissue. Although most of the patients with nasal polyps present eosinophilia, the manifestation of the disease is limited to the airways and this explains why underlying mechanisms should be searched in the tissue and in the cell-to-cell interaction site. The massive sum of some severe inflammatory mediators was found in the micro-environment site formed by the polyp and the surrounding mucosa (21). Cytokine-combined effects and growth factors produced by T infiltrating cells, fibroblasts, epithelial cells or surrounding eosinophilic cells must be responsible for several phases of polyp formation process. GM -CSF Cytokines (Granulocyte - Macrophage Colony Stimulating Factor), IL-3 (interleukin-3) IL-5 (interleukin-5) provide important mechanisms for eosinophil activation and survival (22). Fibroblast- derived GM - CSF seems to play a key role in supporting such eosinophil survival and in macrophage proliferation. (23). TGF - 1 (Transgenic Growth Factor 1 ) is produced by eosinophils in nasal polyps and may contribute to the thickening of local basal membrane, stroma fibrosis and epithelial hyperplasia. (24,25,26).
The effects are apparently complex and sometimes controversial, however, greater quantity of GM - CST and IL-5 in polyps may suggest a prevalence of Th2 cell responses. Cloned T cells of nasal polyps have in average a Th1 standard with high rate of Y-interferon (IFN-Y) interleukin 4 (IL-4), similar to late events of hypersensitiveness reaction and intrinsic asthma.
Eosinophils, however, are not a pre-requirement for the development of nasal polyp. In patients with secondary polyps and cystic fibrosis or chronic maxillary rhinosinusitis due to dental infection, neutrophils are the most numerous inflammatory cells. Regardless some histological differences between eosinophilic polyps and polyps associated with cystic fibrosis, the basic structure is similar in both types. This suggests that in diseases with very different etiology, pathogenic similar mechanisms should rule nasal mucosa response (common final route). Persistent inflammatory state is a common factor in all forms of NP.
The differences between our results and the results obtained by Davidsson and Hellquist(4) could show some variability between studied populations. The sample of seventeen cases in our study does not allow us to extrapolate results as significant for a population.
It may be suggested that the several histological types of polyps found would be distinct evolution stages of the same disease. The existence and mainly the sustained local factors such as chronic bacterial infection may result in qualitative changes in local inflammatory response leading to more chronic histological patterns such as those found in fibrous-inflammatory polyp. This hypothesis is reinforced by the fact that in this study all patients had chronic history of infectious disease before they had undergone surgery and their specimen collection was studied.
CONCLUSIONS
1. Patients with NP had positive skin test rates equal or higher than those of the population in general, and it may suggest a likely relation between polyposis and allergy;
2. There are no histological or ultra structural differences between polyps of allergic and non-allergic patients suggesting that allergy is a contributing factor, but not a primordial one in NP pathophysiology;
3. Polyp-lined epithelium most commonly found was normal respiratory epithelium;
4. Fibrous-inflammatory polyp: Characteristics of inflammatory infiltrate, specially high level of fibroblasts and fibrosis, may suggest that chronic inflammatory process is the only factor responsible for the morphological changes described;
5. Eosinophilic Polyp: None was found. Chronic status of patients included in this study could be a plausible explanation, since presence of edema and eosinophilic infiltrate typical of this kind of polyp would occur in the initial steps of polyp genesis;
6. Fibroid Polyp: Likewise, the presence of chronic inflammation could be related to morphological findings observed, meaning that dense fibrosis could indicate that an inflammatory process is near to be cured;
7. Based on these results we may conclude that polyp genesis in NP is dependent on inflammatory process and such process probably evolves from initial stage to more chronic states. Several categories described by Davidsson and Hellquist(4) could mean stages of an ongoing and evolving process of transformation related to chronic inflammatory process.
REFERENCES
1. CAPLIN, I.; HAYNES, J.T.; SPAHN, J. Are nasal polyps an allergic phenomenon? Ann Allergy, 29: 631-634, 1971.
2. NORLANDER, T.; BRÖNNEGARD, M.; STIERNA, P. The relationship of nasal polyps, infection and inflammation. American Journal of Rhinology, 13(5): 349-355, 1999.
3. BERNSTEIN, J.M.; GORFIEN, J.; NOBLE, B. Role of allergy in nasal polyposis: a review. Otolaryngol. Head. Neck. Surg Vol 113 no 6: 724-732, 1995.
4. DAVIDSSON, A.; HELLQUIST, H.B. The so-called "allergic" nasal polyp. Orl J. Relat. Spec. SS: 30-35, 1993.
5. JAMAL, A & MARANT, AGD. Atopy and nasal polyposis. J. Laryngol. Otol. 101: 355-358, 1987.
6. GRANSTROM, G.; JACOBSSON, E.; JEPPSSON, P.H. Influence of allergy, asthma and hypertension on nasal polyposis. Acta Otolaryngol. 492: 22-27, 1992.
7. VOEGELS, R. - Tese de doutorado- Universidade de São Paulo. 1998.
8. WONG, D. & DOLOVICH, J. Blood eosinophilia and nasal polyps. Am. J. Rhinology. 6: 195-198, 1992.
9. DRAKE- LEE, A.; LOWE, D.; SWANTSON, A.; GRACE, A. Clinical profile and recurrence of nasal polyps. J. Laryngol. Otol., 98: 783-793, 1984.
10. GERGEN, P.G.; TURKELTANG, P.C. The association of allergen skin test reactivity and respiratory disease among whites in the U.S. population. Arch. Intern. Med. 151: 487-492, 1991.
11. KEITH, P.K.; CONWAY, M.; EVANS, S.; EDNEY, P.; JENNINGS, B; ANDERSSON, B.; DOLOVICH, J. A double-blind comparison of intranasal budesonide dry powder vs. placebo in nasal polyposis. J. Allergy Clin. Imunol. 95: 204 (abstract), 1995.
12. STAMM, AC. Microcirurgia Naso-Sinusal. Editora Revinter Ltda. Rio de Janeiro, 1995, 436 pp.
13. RUHNO, J.; HOWIE, K.; ANDERSON, M.; ANDERSSON, B.; VANZIELEGHEM, M.; HITCH, D.; LAPP, P.; DENBURG, J.; DOLOVICH, J. The increased number of epithelial mast cells in nasal polyps and adjacent turbinates is not allergy-dependent. Allergy. 45: 370-374, 1990.
14. HAMILOS, D.L.; LEUNG, D.Y.M.; WOOD, R.; CUNNINGHAM, L.; BEAN, D.K.; YASJRUEL, Z.; SCHOTMAM, E.; HAMID, Q. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J. Allergy Clin. Immunol. 96: 537-544,1995.
15. PARK, H.S.; KIM, H-Y.; NAHM, D.H.; PARK,K.; SUH, K-5; YIM, H.E. The presence of atopy does not determine the type of cellular infiltrate in nasal polyps. Allergy and asthma proc. 19: 373-377, 1998.
16. MYGIND, N.; BRETLAN, P.; SORENSEN, H. Scanning electron microscopic studies of nasal polyps. Acta Otolaryng. 78: 436-443, 1974.
17. LARSEN, P.& TOS, M. Nasal polyps. Epithelium and goblet cell. Density. Laryngoscope, 99: 1274-1280, 1990.
18. CAUNA, N.; HINDERER, K.H.; MANZETTI, G.W.; SWANSON, E.W. Fine structure of nasal polyps. Ann Otol. 81: 41-57, 1972.
19. MYGIND N. BRETLAU, P. Scanning electron microscopic studies of the human nasal mucosa in normal persons and in patients with perennial rhinitis. I: Cilia and Microvilli. Acta Allergol. "Kbh" 28-29 - (1974) Ibid. II: Secretion. Acta Allergol "Kbh" in press.
20. NORLANDER, T., WESTRIN, K.M.; FUKAMI, M.; STIERNA, P.; CARLSÖÖ, B. Experimentally induced polyps in the sinus mucosa: A structural analysis of the initial stages. Laryngoscope, 106: 196-203, 1996.
21. DOLOVICH, J.; OHTOSHI, T.; JORDANA, M.; GAULDIE, J.; DENBURG, J. Nasal polyps: local inductive microenvironment in the pathogenesis of inflammation. Copenhagen: Munksgaard 233-241, 1990.
22. JORDANA, M.; DOLOVICH, J.; OHNO, I. et al. Nasal polyposis: A model for chronic inflammation. Asthma and Rhinitis. Busse W. Holgate S (Eds.) Blackwell Science, 156-164, 1995.
23. VANCHERI, C.; OHTOSHI, T.; COX, G. Neutrophilic differentiation induced by human upper airway fibroblast-derived granulocyte/macrophage colony-stimulating factor (GM-CSF). Am. J. Resp. Cell. Mol. Biol. 4:11-17, 1991.
24. ELOVIC, A.; WONG, D.; WELLER, P. et al. Expression of transforming growth factors- and -1 messenger RNA and product by eosinophils in nasal polyps. J. Allergy Clin. Immunol 93:864-869, 1994.
25. ALLEN, J.S.; EISMA, R.; LEONARD, G.; LAFRENIERE, D.; KREUTZER, D. Interleukin-8 expression in human nasal polyps. Otolaryngol. Head. Neck. Surg. vol 117 no 5: 535-541, 1997.
26. RHEE C.S.; LEE C.H.; MIN, Y.G. Cytokine gene expression in nasal polyps. Ann. Otol. Rhinol. Laryngol. 107: 665-670, 1998.
1 - Master studies under course, Department of Otorhinolaryngology, Medical School of Ribeirão Preto, University of São Paulo - FMRP-USP.
2 - Ph.D., Professor, Department of Surgery and Anatomy, FMRP-USP.
3 - Associate Professor, Department of Otorhinolaryngology, FMRP-USP.
Presented at the II Congresso Triológico de Otorrinolaringologia da SBORL-Goiânia-2001.
Address correspondence to: Prof Dr Wilma T. Anselmo-Lima - Departamento de Oftalmologia e Otorrinolaringologia e Cirurgia de Cabeça e Pescoço do HCFMRP-USP, Avenida Bandeirantes n.º 3900 - CEP: 14049-900 Ribeirão Preto - SP -Tel (55 16) 602-2862- fax: (55 16) 602-2860.