Year: 2003 Vol. 69 Ed. 3 - (2º)
Artigo Original
Pages: 296 to 302
Cytokines profile and HLA typing in tolerant and non-tolerant patients to aspirin with nasossinusal polyposis
Author(s):
Helena M. G. Becker[1],
Roberto E. S. Guimarães[1],
Evaldo Nascimento[2],
Celso G. Becker,
Denise Utsch Gonçalves[3],
Paulo F. T. B. Crosara[4]
Keywords: nasal polyps, aspirin, cytokines, HLA antigen.
Abstract:
Introduction: The eosinophilic infiltration in the nasosinusal polyp associated with intolerance to aspirin is predominant feature. Several mediators play a role in the migration of the eosinophils to the tissues. The IA may be due to overexpression of leukotrienes in genetically susceptible subjects. Objectives: The purpose of this study was to evaluate the cytokine pattern and HLA-A, B and DR typing in subjects with PNS tolerant and intolerants to aspirin. Methods: A transverse study was conducted on 45 patients: 15 patients suffering from eosinophilic PNS and aspirin tolerance (group TA); 15 from eosinophilic PNS associated with aspirin intolerance, the latter manifested by bronchospasm (group IA), and 15 without PNS who had nasal septum deviation (control group). Cytokine pattern (IL-2; IL-4; IL-5; IL-6; IL-8; IL-10; IFN-g and TNF-a) was evaluated in samples from the nasal polyp or midlle turbinate mucosa (control group) of the patients using reverse transcription-polymerase chain reaction (RT-PCR). HLA-A, B and DR typing was performed using the serum microcytotoxicity test or by DNA amplification using polymerase chain reaction (PCR). Results: mRNA expression for interleukines 4, 5, 6, 8, 10, IFN-g and TNF-a was similar in the three groups. mRNA expression for IL-2 was associated with IA. Patients with antigens A11, B49, DR15 and DR13 had a higher likelihood of developing PNS not-related to intolerance to Aspirin, whereas patients with DR17 had a higher likelihood of developing PNS associated with intolerance to Aspirin (Aspirin Triad). Conclusion: PNS associated with intolerance to Aspirin (Aspirin Triad) shows a significant association with HLA- DR17 and IL-2, suggesting a TH1-lymphocyte-activation pattern.
![]()
INTRODUCTION
The aspirin triad - nasosinusal polyps, asthma and Intolerance to Aspirin (IA) were first described by Widal and Samter1,2. IA primary symptoms are bronchospasm occurring also after ingestion of other NSAID (non-steroid anti-inflammatory drugs), cyclooxygenase inhibitors (COX)-1, food dyes and additives, and alcohol.
Intolerance to aspirin and to other NSAID associated with NSP is not IgE-mediated and bronchospasm is three to four time more frequent than hives/angioedema, contrarily to what is found in the population in general 3,4.
The aspirin triad is relatively common affecting 2-3% of all adults with asthma. Its prevalence increased from 8 to 28% when oral aspirin challenge test was performed in patients with difficult to control asthma that generally required steroid therapy. The triad also sustains developmental course regardless of the avoidance of using aspirin and even drugs that could induce cross reaction 3,5.
IA prevalence ranges from 5% to 35% considering NSP patients and has been reported in up to 80% of acute and recurrent cases 5-7.
The most accepted theory for the pathophysiology of intolerance to aspirin suggests that in sensitive individuals Aspirin and NSAID could present an inhibiting pharmacological effect on prostaglandin synthetase-cyclooxygenase activity (COX) that would inhibit COX-1 and COX-2 in all cells resulting in disruption of prostaglandin synthesis (PG) E2 and its modulating effects in inflammatory cells and over expression of cysteinyl-leukotrienes (Cis-LT) through orientation to lipooxygenase route (LOX). Leukotrienes are bronchoconstrictors and chemotactic substances. Some evidence indicates that NSAID-highly selective inhibitors of COX-2 are well tolerated by these patients, revealing that COX-1-inhibition triggers bronchospasm in sensitive patients. LTC4-synthase is the terminal enzyme in Cis-LT production, and is expressed in mast cells, primarily, eosinophils in most of the patients with aspirin-induced asthma (AIA). The allele variant of LTC4-synthase that increases enzyme transcription seems to be associated with AIA. Eosinophil infiltration in respiratory mucosa seems to be AIA key symptom, and eosinophils are the major producers of LT. Eosinophil Cationic Protein (ECP) release increases aspirin challenge. IL-5 expression in respiratory route, known to be involved in eosinophil recruiting, activation, maturation and perpetuation is increased in AIA8,9.
Eosinophil development and differentiation in bone marrow involves GM-CSF, IL-3, and IL-5 cytokines. The latter is more important in eosinophil differentiation and production, as well as in release of those eosinophil already developed in bone marrow for circulation. Eosinophil chemotactic factors directed to inflammatory sites are multiple and include lipid mediators, complement components, cytokines and chemokines. 10
Cytokines play a key role in biological processes regulation namely, cell growth and activation, chemotaxis, inflammation, immunity, tissue repair, fibrosis, and in morphogenesis. Immune response could be assessed according to the profile CD4+ cytokine-secreted lymphocytes, therefore IL-2, IL-12 and IFN- are involved in Th1 immune response and there is IL-3, IL-4, IL-5 and IL-10 secretion in Th2 response. Th1 cells play a role in cytotoxicity effect and inflammation and are involved in the host defense system against viral, microbial and neoplastic diseases. "Th2" cells act primarily in humoral immune response, producing antibodies in atopic reactions and defending the host against parasitic diseases.
The comparison of cytokine expression between allergic and non-allergic patients revealed the existence of different mechanisms involved in build up of eosinophils in these patients. In allergy, build up mechanisms of eosinophil involve the production of IL-3, IL-4, IL-5 cytokines and GM-CSF, type Th2. In non-allergic eosinophilic NSP associated with IA there is the production of IL-3 and GM-CSF and IFN- regardless of IL-4, IL-5, being IFN- the most noticeable cytokine in these groups, what suggests the hypothesis of Thl predominance. Therefore, IL-4 and IL-5 would have their importance restricted to the allergic process 3,12. Subsequently, however, Min et al.13, Bachert et al.14 and Lee et al.15 did not find any significant differences between IL-4, IL-5 and IFN-y concentration in patients with allergic and non-allergic polyps, suggesting that the allergy did not play a significant role in nasal polyp pathogenesis.
The theory that better explained eosinophil increase in nasal polyps was well described by Jankowski16: eosinophil could secret several cytokines, particularly IL-3, IL-5 and GM-CSF. These cytokines prolong in vitro eosinophil survival, increase several metabolic functions and are also involved in tissue migration of these cells. Eosinophil accumulation in lamina propria resulted in release of wide range of cytokines, basic granule-derived proteins (MBP, ECP, EDN, EPX) and lipid mediators, causing inflammation and edema. Subsequently, IL-5 was reported by several authors a the major cytokine responsible for nasal polyp eosinophilia, regardless of allergic process, confirming that the delay of eosinophil apoptosis in the stroma of polyps was mediated by IL-5 and not by GM-CSF, which emphasized the autocrine stimulation of IL-5 in its activation and survival inside the tissue. 13-15,17,18
The abundance of eosinophils in nasal polyps could be explained by the increase in eosinophil migration to the tissues and by the increase in survival level of these cells, but the pathological mechanism with which such eosinophils contribute to tissue damage, inflammatory process and polyp formation still remains unclear. The use of different testing techniques - in situ hybridization, immunohistochemistry, ELISA and RT-PCR in nasal polyp biopsy with no nomenclature standardization, mainly for NSP etiology, has lead to different conclusions regarding the participation of such cytokines, hindering direct comparison. 14
In the utilization of RT-PCR and Southern Blot to analyze patients with nasal polyp with IA, Simon et al.17 detected mRNA for IL-5, IL-1 , IL-8 and TNF- in all polyps, being that mRNA for IL-3 and IFN- were detected only in some cases.
Min et al.13 analyzed allergic and non-allergic patients with PCR and no differences were found in mRNA expression and in the average density of the positive band ray for IL-4, IL-5 and IFN- . Nonetheless, mRNA expression for IL-4 and IL-5 was more frequent in non-allergic polyps than in the nasal concha mucosa.
The cytokines IL-4, IL-5, IL-6, IL-8, TGF- and IFN- were detected in allergic and non-allergic polyps and occasionally in the nasal mucosa15. The authors concluded that all studied cytokines had a role in the pathogenesis of nasal polyp, regardless of the presence or absence of allergy, and that eosinophilia would depend on IL-4 and IL-5, but the expression of the two cytokines could be explained by another immune mechanism and Type I immune response.
Immune genetic evaluations in human beings suggested that sensitivity to genetic diseases is HLA antigen-related. Controversial results in the association between HLA antigen class I and II were reported by Luxenberger et al.19, who did not find in positive association between antigen A1, B8 and IA. Nonetheless, the association between A74 and NSP antigen had statistical significance.
Mullarkey et al.20 detected a significant increase of HLA-DQw2 frequency in patients with AIA, but Lympany et al.21 did not confirm this association, but observed an increase in the frequency of the allele DPB1*1401. Next, Dekker et al.22 detected a strong association between AIA and HLA-DPB1*0301, which differed from DPB1*1401 by only one amino acid, what suggests that these alleles have a similar function. Molnar-Gabor et al.23, using PCR, demonstrated that patients with HLA-DR7-DQA1*0201 and DQB1*0202 present two to three times increased risk of developing NSP. Additionally, they also found a statistical significant relation between IA and HLA-DR7, suggesting that IA and NSP represented a particular form of NSP.
The objective of this study was to analyze cytokine profile (RT-PCR) and the frequency of Class I and II HLA in patients with eosinophilic nasal polyps tolerant and intolerant to aspirin.
MATERIAL AND METHOD
Forty-five non-allergic adult patients of the Service of Otorhinolaryngology, UFMG Clinical Hospital: Group IA - 15 patients with IA (Intolerant to Aspirin) with eosinophilic NSP and clinical records of IA with bronchospasm symptoms, being 9 females aged 19 to 58 years (mean age was 37.27 years); Group TA - 15 patients with TA with eosinophilic NSP and absence of IA (8 patients were female, aged from 27 to 69 years, mean age of 44.6 years). Control group - 15 patients without NSP, eosinophilic mucosa and absence of IA, with nasal septum deviation of traumatic cause (2 patients were female aged 17 to 69 years, mean age of 39.87 years).
All patients signed an informed consent term for the study, with protocol approved by the Ethics Committee on medical research.
The presence of nasal allergy was excluded by clinical history and skin test. The presence of NSP was confirmed by nasosinusal endoscopy. Nasal polyp eosinophilia (>30%)24 was found after biopsy and fragment staining with HE.
Intolerance to Aspirin evidenced by bronchospasm was diagnosed by clinical history of patients in-group IA. Oral challenge test was carried out in those patients with former history of negative IA (TA and control groups) to confirm the absence of IA. A reduction in forced expiratory volume equal or higher than 20% in one second was considered positive. Bronchodilator was not used 72 hours before aspirin challenge test.
Before carrying out skin test in patients, nasal biopsy to analyze cytokine profile and histopathology was performed, but topical or systemic use of steroids and anti-histaminic drugs were not used for at least 30 days before the test.
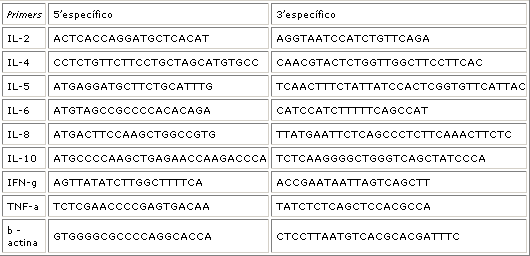
Cytokine profile was studied in the fragment of middle concha (control group) or nasal polyp (groups IA and TA), and were then prepared to obtain RNA and subsequent electrophoresis analysis through reverse reaction of polymerase chain (RT-PCR), with analysis of the transcriptions: IL-2; IL-4; IL-5; IL-6; IL-8; IL-10; IFN- ; TNF- and -actin (Table 1).
HLA-A, B and DR typing was carried out by means of serum test (purified leukocytes) with microtoxicity to HLA A and B and by amplification of DNA by polymerase chain reaction with specific initiator method (SSP-PCR) for HLA DR. Gene frequency of alleles HLA-A, B and DR published in individuals from mixed population from Belo Horizonte and Rio de Janeiro were used as controls 25, 26
Statistical analysis employed Pearson Chi-square with level of significance of 5%.
RESULTS
Cytokine expression of polyps was analyzed in 12 patients for the IA group, 14 patients of TA group and in the middle turbinate mucosa in 13 patients of the control group (Figure 1). Losses occurred during preservation and processing of biopsies.
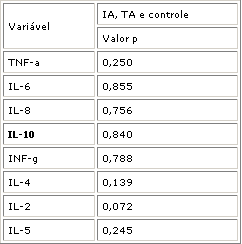
The comparison of cytokine expression (TNF- , IL-6, IL-8, IL-10, INF- , IL-4, IL-2 and IL-5) did not evidence a statistically significant difference (p>0.05) among the three groups. Nonetheless, at the level of 7% there was a difference in the expression of IL-2 (p=0.072) (Table 2), resulting from the differences between TA and IA groups (p= 0.022), in which IL-2 was present in 92% of the IA cases and in 50% of the TA cases. The analysis between the IA group and the control group and TA group were not statistically significant (p>0.05).
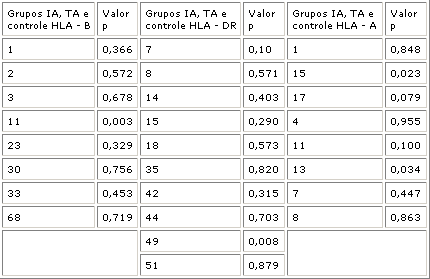
Non-identified antigens were excluded. Frequencies compared were as follows: HLA-A among IA (n=25), TA (n=30) and control (n=190) groups; HLA-B among IA (n=28), TA (n=29) and control (n=190), and HLA-DR among IA (n=29), TA (n=27) and control (n=349) (Table 3). All statistical differences below 10% were analyzed and it was observed that:
- The A11 antigen frequency did not present any statistical difference between IA and TA (p=0.337) and between IA and the control group (p=0.100). There was difference, however, between TA and the control groups (p=0.001; OR=7.40; IC=1.95 - 28.11), in which A11 antigen was present in 17% of the cases in TA group and only in 3% of the cases in the control group.
- The B49 antigen frequency did not present any differences in comparisons with IA and TA (p=0.337) and between IA and the control (p 0.10) group, but there was significant difference between TA and the control group (p = 0.002; OR=7.44; IC=1.69-32.58), in which B49 was found in 13% of the cases of the TA group and in only 2% of the cases in the control group.
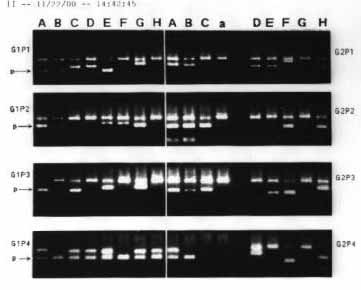
Figure 2 shows the typing of HLA-DR antigen in two patients due to PCR. Analyzing significant statistical associations (p<0.10) involving HLA-DR 15, 13 and 17, the following was found: a) There was difference between TA group and control group (p = 0.023; OR=3.94; IC=1.32-11.82), in which HLA-DR15 was present in approximately 19% of the cases in group TA and in only 5% of the cases in the control group. b) There was difference between TA group and the control (p = 0.009; OR=3.24; IC=1.25-8.38), in which HLA-DR13 antigen was present in approximately 26% of the cases in TA group and in only 10% of the cases in the control group. c) There was some difference between IA and control group (p = 0.035; OR=2.99; IC=1.02-8.75), in which DR17 antigen was present in approximately 17% of the cases in IA and in only 75 of cases in the control group.Table 1. Sequence of primers used in RT -PCR for cytokines.Table 2. Comparison between the proportion of patients and the expression of cytokines in groups IA, TA and the control group.Table 3. Comparison of HLA-A, B and DR antigen frequency in groups IA, TA and the control group.Figure 1. Summary of cytokine expression: A - TNF- , B - IL-6, C - IL-8, D - IL-10, and - INF- , F - IL-4, G - IL-2, H - IL-5, per cell in polyps of patients of groups IA and TA and in nasal mucosa control group patients. Product of positive amplification of dark rectangles. NR = not performed. % indicates the number of positive cells.Figure 2. Reverse transcription in polymerase chain reaction (RT-PCR) representing the products of expression of TNF- (A), IL-6 (B), IL-8 (C), L-10 (D), INF- (E), IL-4 (F), IL-2 (G), IL-5 (H) cytokines and = actin, per cell present in the polyps of patients (P1,2,3,4) of groups (G1,2) I-IA and II-TA. Fragments stained with ethidium bromide at 2% agarose . RT- Reverse Transcriptase
DISCUSSION
By using RT-PCR, the mRNA expression for IL-5 was found in all allergic and non-allergic polyps and in the IA association and was not found in the mucosa of the nasal conchae15, 17. In the present study the frequency of the mRNA expression for IL-5 was similar in IA (58.3%), TA (85.7%) and control (61.5%) groups, and contrary to the literature, IL-5 expression was not observed in all polyps.
The mRNA expression for IL-4, IFN- , IL-6 and IL-8 was observed in all allergic and non-allergic polyps 13, 15 and was not detected in association with IA17. In this study, the frequency of mRNA expression for IL-4 was similar in IA (50%), TA (85.7%) and control (61.5%) groups; the frequency of mRNA expression for IFN- was similar in IA (66.7%), TA (57.1%) and control (69.2%) groups and the frequency of the expression of IL-6 and IL-8 were not different between the IA, TA and control group, nor its expression was observed in all patients of the same group.
In this observation, the only cytokine expressed in all patients with IA was TNF- , a finding also observed by Simon et al.17.
The frequency of mRNA for IL-10 using RT-PCR was not commonly described in the world literature. This expression was not different between IA, TA and control group.
A positive association between IL-2 expression in IA and in relation to TA group was found in this study. Weller10 reported that eosinophils expressed IL-2 receptors and this cytokine mediated the migratory eosinophil response. The findings of this study suggest the involvement of a Th1 type response in association with IA similarly to that described by Schapowal3 and Hamilos11 and that IL-2 may play an important role in NSP associated with IA.
Significant increase of HLA-A, B and DR frequency in patients with AIA NSP is controversial 21-23. Statistical significant difference was not found in patients of this study regarding typing of HLA-A, B and DR in comparison between groups IA and TA. Nonetheless, a positive association between TA and antigens namely A11 (p=0.001), B49 (p=0.002), DR15 (p=0.023), DR13 (0.009) and between IA group and DR17 (p=0.035) antigen compared to the control group (Brazilian Population). Patients with A11, B49, DR15 and DR13 antigens showed higher likelihood of presenting IA non-related NSP whereas DR17 patients presented increased likelihood of having NSP and AI (Aspirin triad). These associations were not described in the literature.
The importance of studying the mediators involved in the pathogenesis of NSP and the attempt to elucidate the increase in HLA frequency (genetic susceptibility) in a determined group of NSP patients IA (intolerant to aspirin) were based on the following facts: although the international literature considered rhinosinusitis the only disease defined as "inflammatory disease of nose and paranasal sinuses that persists and causes symptoms for more than three months with thickening of nasal mucosa and nasal polyp as the last stage of chronic inflammation" 27, several etiologies are associated with this definition. Chronic fungal rhinosinusitis infection, intolerance to aspirin, mucociliary diskinesis, immune deficiency, cystic fibrosis with or without eosinophilic infiltration of mucosa, could be quoted as examples. Patients with non-eosinophilic polyps, such as cystic fibrosis, recessive autonomic disease, chronic inflammation of mucosa resulting from thickening of mucus due to mutations in cystic fibrosis transmembrane conductance regulator (CFTR) gene and subsequent edema of this mucosa followed by polyp formation. Aspirin and anti-inflammatory drugs are known as the triggering factor for eosinophil migration (major leukotriene producers) in the respiratory mucosa of patients genetically susceptible that presented LTC4 synthase expression, a final enzyme in the production of bronchoactive leukotriene. Fungi detected in nasal mucus of almost all patients with chronic rhinosinusitis and also in healthy individuals are pointed as being responsible for the migration of the eosinophils from the mucosa to the mucus, in an attempt to destroy them after the release of their protein content in their granules 27. How can we explain that the same fungi are able to attract eosinophils to nasal mucosa in some patients since healthy individuals also have fungi in their noses? Would eosinophils of patients have some abnormality (genetic predisposition?) that makes them migrate to nasal mucosa (attracted by fungi) and then enables the manifestation of self-sustained eosinophil inflammatory response? Why fungal eosinophilic rhinosinusitis is usually unilateral? Why children have low prevalence of nasosinusal polyposis? There is not a single etiologic factor that could explain the pathogenesis of nasosinusal polyposis such as a single disease: inflammation is still the primary factor, regardless of the etiology. The importance of this study is that it was an attempt to know the role of some inflammatory mediators in a specific group of patients with chronic rhinosinusitis (with nasosinusal polyposis and intolerance to aspirin) and that it tried to detect a genetic pattern of immune response (HLA) for such patients.
CONCLUSION
The expression of mRNA for interleukins 4, 5, 6, 8, 10, IFN- and F- was similar in nasal polyps of patients with nasosinusal eosinophilic polyposis tolerant and intolerant to aspirin. The mRNA expression for IL2 was associated with intolerance to aspirin suggesting a TH1 profile.
Patients with antigen A11, B49, DR15 and DR13 had increased likelihood of developing nasosinusal polyposis non-related to IA whereas patients with DR17 presented increased likelihood of developing nasosinusal polyposis associated with intolerance to aspirin.
REFERENCES
1. Widal F, Abrami P, Lermoyez J. Anaphylaxie et idiosyncrasie. Presses Med 1922;18:189-93.
2. Samter M, Beers RF. Concerning the nature of intolerance to aspirin. J Allergy 1967 Nov;40(5):281-93.
3. Schapowal AG, Simon HU, Schmitz-Schumann M. Phenomenology, pathogenesis, diagnosis and treatment of aspirin-sensitive rhinosinusitis. Acta Otorhinolaryngol Belg 1995;49(3):235-50.
4. Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc 1996;17(5) Sep/Oct:181-236.
5. Kramer MF, Rasp G. Nasal polyposis: eosinophils and interleukin-5. Allergy 1999; 54:669-80.
6. Schiavino D, Nucera E, Milani A, Ninno MD, Buonomo A, Sun J, Patriarca JSG The aspirin disease. Thorax 2000;55 Oct (Supl 2):66-9.
7. Moneret-Vautrin DA. La physiopathologie de l`intolérance à l`aspirine. In: Freche CL, Fontane JP, Peynegre. La polypose naso - sinusinne. Paris: Europeenne; 2000. p. 39-45.
8. Sanak M, Szczeklik A. Genetics of aspirin induced asthma. Thorax 2000;55 Oct. (Supl 2):45-7.
9. Szczeklik A, Nizankowska E, Mastalerz L, Szabo Z. Analgesics and asthma. Am J Ther 2002:9(3):233-43.
10. Weller PF Human eosinophils. J Allergy Clin Immunol 1997;100:283-87.
11. Hamilos DL. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol 1995:96(4):537-44.
12. Voegels RL. Polipose nasal: estudo da correlação entre as interleucinas 1, 3, 4 e 5 e a molécula de adesão VCAM-1 com a presença ou não de alergia. São Paulo: Faculdade de Medicina da USP, 1998. 100p. (Tese, Doutorado em Otorrinolaringologia).
13. Min YG, Lee CH, Rhee CS, Kim KH, Kim CS, Koh YY, Min KU, Anderson PL Inflammatory cytokine expression on nasal polyps developed in allergic and infectious rhinitis. Acta Otolaryngol Stockh 1997;117(2):302-6.
14. Bachert C, Gevaert P, Holtappels G, Cuvelier C, Van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol 2000;14(5):279-90.
15. Lee CH, Rhee CS, Min YG. Cytokine gene expression in nasal polyps. Ann Otol Rhinol Laryngol 1998;107(8):665-70.
16. Jankowski R. Eosinophils in the pathophysiology of nasal polyposis. Acta Otolaryngol Stockh 1996;116(2):160-3.
17. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 1997;158(8):3902-8.
18. Rudack C, Bachert C. Cytokines and chemokines in paranasal sinus diseases. Laryngorhinootologie 1999;78(9):481-90.
19. Luxenberger W, Posh U, Berghold A, Hofmann T, Lang-Loidolt D. HLA patterns in patients with nasal polyposis. Eur Arch Otorhinolaryngol 2000;257(3):137-9.
20. Mullarkey MF, Thomas PS, Hansen JA, Webb DR, Nisperos B. Association of aspirin-sensitive asthma with HLA-DQw2. Am Rev Respir Dis 1986;133(2):261-3.
21. Lympany PA, Welsh KI, Christie PE, Schmitz-Schumann M, Kenemy D, Lee TH. An analysis with sequence-specific oligonucleotide probes of the association between aspirin-induced asthma and antigens of the HLA system. J Allergy Clin Immunol 1993;92(1):114-23.
22. Dekker JW, Nizankowska E, Schmitz-Schumann M, Pile K, Bochenek G, Dyczek A, Cookson WOCM, Szczeklik A. Aspirin-induced asthma and HLA-DR1 and HLA-DPB1 genotypes. Clin Exp Allergy 1997;27(5):574-7.
23. Molnar-Gabor E, Endreffy E, Rozsasi A. HLA -DRB1, -DQA1, and -DQB1 genotypes in patients with nasal polyposis. Laryngoscope 2000;110(3):422-45.
24. Ingels K, Durdurez JP, Cuvelier C, Cauwenberge PV. Nasal biopsy is superior to nasal smear for finding eosinophils in nonallergic rhinitis. Allergy 1997;52(3):338-41.
25. Willians A, Meenagh A, Darke C, Acosta, A, Daar AS, Gorodezky C, Hammond M, Nascimento E, Middleton D. Analysis of the distribution of HLA-B alleles in populations from five continents. Hum Immunol 2001;62:645-50.
26. Middleton D, Willians A, Meenagh A, Daar AS, Gorodezky C, Hammond M, Nascimento E, Briceno I, Perez MP. Analysis of the distribution of HLA-A alleles in populations from five continents. Hum Immunol 2000;61:1048-52.
27. Braun H, Buzina W, Freudenschuss K, Beham A, Stammberger H. Eosinophilic fungal rhinosinusitis: a common disorder in Europe? Laryngoscope 2003;113(2):264-9.
1. Joint Professor, Department of Otorhinolaryngology, Ophthalmology and Speech and Language Pathology and Audiology, Medical School, Federal University of Minas Gerais.
2. Joint Professor, Department of Parasitology, Institute of Biological Sciences, Federal University of Minas Gerais.
3. Undergraduate in Medicine, Federal University of Minas Gerais. Resident in Otorhinolaryngology, Minas Gerais.
4. Otorhinolaryngologist, Ph.D. studies under course, Medical School, Federal University of Minas Gerais.
Affiliation: Department of Otorhinolaryngology, Ophthalmology and Speech and Language Pathology and Audiology, Medical School, UFMG.
Address correspondence to: Helena Maria Gonçalves Becker -# Av. Pasteur 88, 4º andar Belo Horizonte MG 30150-290 - Tel/Fax (55 31) 3222-2891 E-mail: hbecker@medicina.ufmg.br
Study presented at 36o Congresso Brasileiro de Otorrinolaringologia (SBORL) in Florianópolis/SC, on November 19 - 23, 2002.