Year: 2003 Vol. 69 Ed. 3 - (9º)
Artigo Original
Pages: 343 to 347
Prospective, randomized, double-blind clinical trial about efficacy of homeopathic treatment in children with obstructive adenoid
Author(s):
Sergio E. Furuta[1],
Luc L.M. Weckx[2],
Claudia R. Figueiredo[3]
Keywords: adenoid, homeopathic treatment.
Abstract:
Objetive: the efficacy and security of homeopathic treatment was investigated on children with obstructive adenoid justifying an operation. Methods: In a prospective, randomized, double-blind clinical trial included 40 children between the ages of 3 to 7 years old, 20 children were treated with homeopathic medication, based in the principle of similarity (Simillimum), and 20 children with placebo. All the children of the homeopathic group/ adenoid, were treated daily with Agraphis nutans 6 CH, Thuya 6 CH and Adenoid 21CH, and the patients of the placebo group received daily placebo medication. The duration of the study of each children was 4 months. The evaluation of the results was clinical, and it was made by questionnaire standard, clinical examination and direct flexible fiberoptic nasopharyngoscopy, in the first and last day of treatment. The criterion of selection was the adenoid that occuped more than 70% of the coanal space. Results: From the group of 20 children treated with homeopathic treatment, 13 did not show any change on the size of adenoid after nasopharyngoscopy, and 7 children had their adenoid decreased; from another group of 20 children that have treated with placebo for 4 months, 11 did not show any change on the size of their adenoid, 4 had their adenoid decreased and 5 had their adenoid increased. The statistical analysis showed a not significant difference (P= 0,069). The clinical evaluation of the patients showed that from the group of 20 patients treated with homeopathy, 17 kept unchanging, with oral breathing and snoring, one patient got better, eliminating the snoring and two were cured, which mean that their oral breathing turned to nasal breathing without snoring. From the group of 20 patients treated with placebo, 17 kept unchanging, one eliminated the snoring and two were cured; and these differences were not statistically significant (P> 0,999). Conclusions: the homeopathic treatment was not efficient in the patients with obstructive adenoid, remaining it surgical indication in 85% of the children. The homeopathic remedies did not provoke adverse events in the children.
![]()
INTRODUCTION
Palatine tonsil and pharyngeal hypertrophy are common causes of frequent pediatric and/or ENT appointment, and surgical recommendation in otorhinolaryngology. In the first half of the century, tonsillectomy and adenoidectomy were recommended for minor symptoms even as a routine surgery for most pediatric patients' diseases from mental retardation to enuresis. 1 In the 60's studies reported many cases of surgery ineffectiveness and surgical recommendation started to be questioned. At that time, some research on the immune role of the structures of the Waldeyer's lymphatic ring resulted in more conservative and stringent surgical indications 1.
Homeopathy was founded by Samuel Hahnemann, a German physician, in 1796 and has been successfully used to prevent and treat palatine tonsil and pharyngeal diseases reducing the number of patients indicated for surgery 2 . In addition, the price of homeopathic drugs is cheaper than that of conventional drugs. Information on the efficacy of homeopathic drugs, however, is scarce in the literature, especially related to proper scientific methods using double-blind, randomized and control methodology.
Homeopathy (Greek homolós= similar + páthos= disease or suffering) is a system of therapeutics based on the law of natural cure Similia similibus curentur (similar cured by similar)3. It is a therapy capable of producing in healthy person symptoms like those of the disease to be treated, obtained by trying a substance in apparently healthy and sensitive individuals. This substance, a homeopathic drug named Simillimum or "background drug", is prepared in ultra diluted formulations. 4
The principles of Homeopathy are: 1. Law of similarity; 2. Experiments in healthy individuals (the experimentation of drugs should not be carried out in sick patients or in animals, but in healthy and sensitive individuals; the set of symptoms during the experimentation is known as pathogenesis); 3. Single drug (to use a single drug, the Simillimum); 4. Minute dose (diluted and dynamized drug)5.
Homeopathic drugs are not simple dilutions of a substance to the infinite. Each time the drug is diluted, it is vigorously agitated and this mechanism is known as sucussion. It is believed that such agitation "releases" the latent energy of the drug (dynamization). It originates from the three kingdoms: vegetal, mineral and animal. Generally, drug formulation is performed in decimal and centesimal scales. In the decimal scale a basic part of the substance is diluted in 9 parts of an excipient and is agitated (sucussion) 100 times (1D). The excipient generally is an alcohol at 30%. The centesimal dilution is performed with a part of base substance diluted in 99 parts of the excipient and is sucussioned 100 times (1 CH = 1 Hahneman Centesimal).
According to the Brazilian Homeopathic Pharmacopoeia, an isopathic drug is as drug formulation to be used in homeopathy obtained from biological products, chemically undefined, that could be secretions, excretions, tissues or organs, either pathological or not, products of microbial and allergen nature. According to Costa7, isopathy is a method to cure diseases through its causing agents manipulated upon homeopathic techniques (dynamized). The author advocates the association of homeopathy with therapies using three drugs for the same patient:
a) A drug based on all patient's symptoms, the constituent drug or "background" drug, or similar homeopathic drug.
b) Episode drug or Syndrome drug, or the drug that relates to the acute symptoms, which are more bothersome and typically local symptoms. Typically an specific drug for the physical symptoms within the organicist management.
c) Ethyopathogenic drug or pathophysiological drug of the disease; according to the clinical case, as follows:
c1) Nosodic - Isopathic non-lysate or destroyed drug, and/or specific, dynamized autogenous live drugs.
c2) Allergen or mediating dynamized drugs.
c3) Dynamized organotherapic drugs.
The expression Nosodic (Greek noso: disease) is the drug prepared according to the homeopathy Pharmaco-technique with vegetal and animal pathological products. In France such products are known as Biotherapic drugs 7.
This double-blind randomized clinical trial aimed at assessing the efficacy and safety of homeopathic drugs in pediatric patients with surgical indication for adenoidectomy due to obstructive pharyngeal tonsil.
METHOD
Forty patients were selected from the outpatient facility of the Department of Pediatric ORL of Escola Paulista de Medicina/ Federal University of Sao Paulo (UNIFESP) and of Hospital São Paulo, from March/2000 through September/2001, patients aged 3 to 7 years old with surgical indication due to obstructive adenoid, waiting for surgery vacancy. Patients with systemic diseases and immune deficiency were excluded from the trial. Obstructive adenoid in mouth breathers, with or without sleep snoring and/or apnea, was characterized and evaluated using nasofibroscopy performed by an ENT doctor. We considered obstructive adenoid if the nasofibroscopy showed that it filled more than 70% of the choanal lumen 8.
Samples were split and randomized in 2 double-blind groups:
Group I - Twenty pediatric patients with obstructive adenoid diagnosis that were administered homeopathic treatment for 4 months.
Group II - Twenty pediatric patients with obstructive adenoid diagnosis that were administered placebo for 4 months.
Parents or tutors of the patients were informed about the objective of the study and provided their informed consent in writing. This trial was approved by the Ethics Committee of Research Studies, Federal University of Sao Paulo (UNIFESP) - EPM.
The treatment consisted of the administration of three drugs for all group I patients, based on the personal experience of Costa7 & Linhares2:
1) The drug based on the conformation of the patient, also known in homeopathy as "Simillimum" or "background", customized based on physical and mental similarities of the patient obtained in patient's examination, with 30 CH (potency) in single dose, followed up every 4 weeks for 4 months. A computer software (Repertório Homeopático Digital II9) was used to support the calculation of the Simmillimum drug.
2) The drug based on organic, local and adenoid features (Agraphis nutans) with 6CH and Thuya 6 Ch (potency), daily administration for 4 months:
3) Isopathic drug, Adenoid 21 CH, daily administration for 4 months.
Placebo was administered to Group II patients as if was a conformational drug and was given in single dose. Placebo was also administered as Agraphius nutans, and as Thuya as if it was the adenoid drug, in daily doses for 4 months. The list of randomized drugs was made by the homeopathic pharmacist responsible for the formulation of the drugs and was released only after the end of the treatment to all patients. The placebo was formulated with 30% alcohol with the same odor, flavor and appearance of the active homeopathic drug. The role of the alcohol was to be a preservative. All drugs used in this trial were in compliance with the Brazilian Homeopathic Pharmacopoeia (1997)6.
Clinical evaluation consisted of a standard questionnaire and follow-up on a monthly basis for four months. All patients underwent ENT evaluation that was included in the ENT examination (oroscopy, anterior rhinoscopy and otoscopy) and nasofibroscopy on the first and last day of the treatment and it was performed by an Otorhinolaryngologist of the Department of Pediatric Otorhinolaryngology of Escola Paulista de Medicina/UNIFESP. By the end of the study, all patients who still had indication for surgery were referred to it.
The statistical method applied was Fisher's exact test or it was extended in tables larger than 2X2, and significance rate was set as 0.05 or 5%, with significant values highlighted with (*).
RESULTS
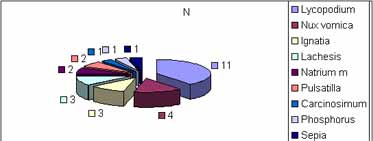
Pediatric patients were selected as follows: 17 girls (43%) and 23 boys (57%) aged from 3 to 7 years old. All patients followed the treatment with no interruptions or dropout. According to the similarity principle, homeopathic drugs were prescribed individually: Lycopodium, Nux-vomica, Ignatia, Lachesis, Natrum muriaticum, Pulsatilla, Carcinosinum, Phosphorus and Sepia (Graph 1).
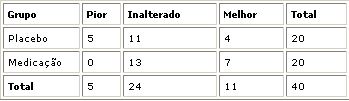
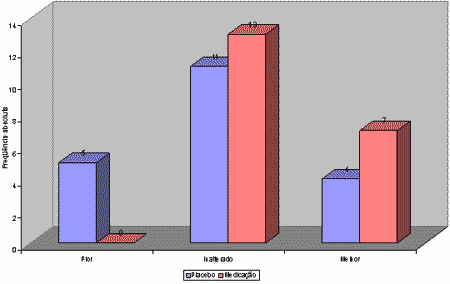
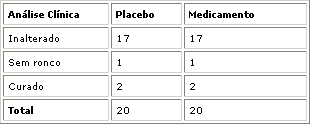
Nasofibroscopy results did not show any variation in adenoid size (unchanged result) in 13 patients of group I (homeopathic drug) and in 11 patients of group II (placebo). Adenoid decrease was reported in 7 patients in group 1 (better outcome) and in 4 patients in group II (placebo). Five patients of group II (placebo) presented increase in adenoid size (worse outcome), see Table 1 and Graphs 2, 3, 4. Statistical analysis did not show any significance between group I and II (P= 0.069 or 6.9%).
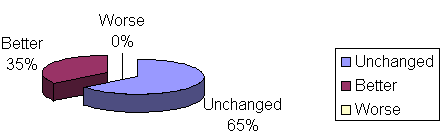
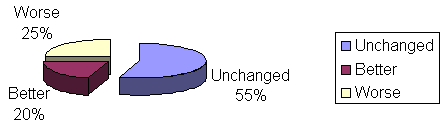
Clinical evaluation of patients considering breathing in group I (homeopathic drug) - 17 patients remained unchanged, with mouth breathing and snoring, 1 patient got better, snoring disappeared, and 2 patients were cured, or breathing behavior changed from mouth breathing to nasal breathing and without snoring, according to Table 2 and Graph 5. Seventeen patients in group II (placebo) remained unchanged, 1 patient improved snoring and 2 were cured. There was no statistically significant difference between groups I and II (P>0.999 or 9.99%).
No adverse effects were reported in patients of group I and II with the use of the medicines.Graph. 1Table 1. Obstructive adenoid / Patient outcome comparing initial and final nasofibroscopy. Fisher's Exact Test: P=0.069 or 6.9%Graph 2. Patient distribution according to outcome and group studied. Initial and final nasofibroscopy evaluation / Obstructive adenoid
Fisher's Exact Test: P=0.069 or 6.9%
Absolute frequency
Placebo/medication
Worse/Unchanged//BetterGraph 3. Outcome of group I patients (homeopathic drug) comparing initial and final nasofibroscopy.Graph 4. Outcome of group II patients (placebo), comparing initial and final nasofibroscopy.Table 2. Outcome of patients / Clinical Evaluation / Obstructive Adenoid.Graph 5. Distribution of patients according to outcome and study group / Clinical Evaluation/ Obstructive Adenoid.
DISCUSSION
Homeopathy has been used as an alternative therapy in obstructive adenoids with the purpose to reduce surgical indication. The challenge in the conduction of a randomized double-blind method in homeopathic studies is its characteristic of individualizing each patient, determining the similarity principle, specific drug ("Simillimum"), regardless of the clinical diagnosis at stake. Homeopathy treats patients as an entire organism, not only the symptom or the disease. Therefore, 9 different homeopathic drugs were selected for the individualized clinical diagnosis of obstructive adenoid. Care to all patients was provided by only one doctor.
Comparison of nasofibroscopy results in the beginning and at the end of the treatment showed that in group I (homeopathic drug) 7 patients (35%) improved and 13 (65%) remained unchanged. In group II (placebo), 4 patients (20%) improved, 11 patients (55%) remained unchanged and 5 (25%) got worse. Results were not statistically significant. Clinical evaluation of patients in groups I and II, with analysis of breathing behavior (oral or nasal) and snoring, was not significantly different. Seventeen patients (85%) of group I and II sustained recommendation for surgery. These results were compatible with those obtained by Friese et al.10 that treated 82 patients aged 4 to 10 years old with obstructive adenoid and recommendation for surgery for three months per patient in a randomized double-blind study. At the end of the treatment 70.7% of the patients treated with placebo and 78.1% treated with homeopathic drug did need surgery, concluding that there was no statistical significance.
CONCLUSIONS
Results obtained with clinical evaluation and nasofibroscopy performed in 40 patients with obstructive adenoid and surgery indication aged 3 to 7 years old that received homeopathic treatment or placebo in a randomized double-blind study with four-month follow up allowed us to conclude that:
1) Homeopathic treatment was not effective in patients with obstructive adenoid and 85% of the patients maintained the indication for surgery.
2) Homeopathic drug did not cause any side effects in patients.
ACKNOWLEDGEMENTS
To Dr Carlos Roberto Dias Brunini, Professor of Homeopathy, for his support and guidance. To Andrea Cristina de Oliveira, Pharmacist of Farmácia Núcleo da Manipulação, for supplying the medicines and placebo list.
To Post-graduate and Specialization Program Students of the Department of Pediatric Otorhinolaryngology, UNIFESP/EPM for consultation and the ENT examinations in this study.
REFERENCES
1. Rosenfeld RM, Green RP. Tonsillectomy and adenoidectomy: changing trends. Ann Otol Rhinol Laryngol 1990;99:187-191.
2. Linhares W. Homeopatia em pediatria. 4a ed. São Paulo: Homeolivros; 2000. 109 p.
3. Hahnemann S. O moderno Organon da arte de curar. Tradução Marcelo Pustiglione. 6ª ed. Alemã. São Paulo: Typus; 2001. 320 p.
4. Kossak-Romanach A. Homeopatia em 1000 conceitos. São Paulo: Elcid; 1984. 27, 170.
5. Eizayaga FX. Tratado de medicina homeopática. Buenos Aires: Marecel; 1992. 400p.
6. Farmacopéia Homeopática Brasileira. 2ª ed. parte I. São Paulo: Atheneu; 1997.
7. Costa RA. Homeopatia atualizada: Escola brasileira. 3a ed. Petrópolis: Vozes; 1988. 78, 80, 94, 145-146.
8. Chami FI. Avaliação nasofibroscópica e radiológica de pacientes com hiperplasia de amígdala faríngea. São Paulo, 1997. [Mestrado] Universidade Federal de São Paulo - Escola Paulista de Medicina. 57 p.
9. Ribeiro Filho A, Bronfman Z. Repertório Homeopático Digital II São Paulo: Organon 2000. CD-ROM.
10. Friese KH, Feuchter U, Moeller H. Die homoopathische behandlung von adenoiden vegetationen. Ergebnisse einer prospektiven randomisierten Doppelblindstudie. HNO 1997;45(8):618-24.
1 - Master in Homeopathic Medicine, School of Healthcare Sciences of São Paulo/ Instituto Brasileiro de Estudos Homeopáticos. Pediatrician, Escola Paulista de Medicina/ UNIFESP.
2 - Full Professor in Otorhinolaryngology, Escola Paulista de Medicina/ Universidade Federal de São Paulo. Head of the Discipline of Pediatric Otorhinolaryngology, Escola Paulista de Medicina/ UNIFESP.
3 - Master in Otorhinolaryngology and Head and Neck Surgery; Doctorate studies in Otorhinolaryngology under course, Escola Paulista de Medicina / UNIFESP.
Discipline of Pediatric Otorhinolaryngology, Escola Paulista de Medicina/ Universidade Federal de São Paulo.
School of Healthcare Sciences of São Paulo / Instituto Brasileiro de Estudos Homeopáticos.
Address correspondence to: Sergio E. Furuta - Disciplina de ORL Pediátrica da UNIFESP - EPM. R. dos Otonis, 674 São Paulo SP 04025-001 - Tel (55 11) 5539-7723 - E-mail: orlped@epm.br
Master thesis submitted to the School of Healthcare Sciences of São Paulo - Instituto Brasileiro de Estudos Homeopáticos, São Paulo/ March 2002. - Paper submitted as poster at 36° Congresso Brasileiro de Otorrinolaringologia, November 2002.