Year: 2005 Vol. 71 Ed. 4 - (22º)
Artigo de Revisão
Pages: 536 to 546
Some considerations about acquired adult and pediatric cholesteatomas
Author(s): Cristina Dornelles , Sady S. da Costa , Luíse Meurer , Cláudia Schweiger
Keywords: cholesteatoma, perimatrix, collagen.
Abstract:
Authors debate about cholesteatomas, from the first time this word was employed, by Muller, in 1838, until the recent updates. They dissert about its definition, etiology and pathology and present basic concepts about its biology. They also make a wide review about pediatric cholesteathoma, its epidemiology and biology, and compare it with adult cholesteatoma. Finally, they describe some articles about ossicle chain erosion and its correlation with cholesteatoma perimatrix, collagen and collagenase.
![]()
The word
The word "cholesteatoma" was used for the first time by the German anatomist Johannes Mueller, in 18381. The roots of this word mean cole - cholesterol; esteado - fat; oma - tumor, that is, a tumor in which we have fatty tissue and cholesterol crystals. Etymologically, this term is completely incorrect, and it is considered the second wrong term in otology (the first one is acoustic neuroma, given that it is in fact a vestibular nerve Schwannoma)2. The use of this denomination is inappropriate because cholesteatoma is originated from the keratinized squamous epithelium of the tympanic membrane and/or external auditory canal, without presence of cholesterol crystals or fat in its structure, in addition to the fact that the tumor nature is completely discussable.
Other suggested denominations were pearl tumor, by Cruveilhier, in 1829; margaritoma, by Craigie, in 1891, epidermal cholesteatoma by Cushing, in 1922, epidermoid by Critchley and Ferguson, in 1928 and keratoma, by Shuknecht, in 1974; all these terms even though they are more appropriate and descriptive, are not employed and the term cholesteatoma is widely used by otologists.
Definition
Cholesteatomas were defined by Friedmann3, in 1959, as cystic structures recovered with stratified squamous cell epithelium, laying over a fibrous stroma of variable thickness, which can contain some elements from the original mucous lining.
Schuknecht4, in 1974, defined them as accumulation of exfoliated keratin inside the middle ear or any pneumatized area of the temporal bone, originated from the keratinized squamous epithelium. Informally, the author stated that the cholesteatoma could be characterized as "skin on the wrong side".
Epidemiology
Annual incidence of cholesteatoma ranges around 3 in 100,000 in children and 9 in 100,00 in adults, and it is more predominant in male 5,6.
Epidemiological data show high prevalence of cholesteatoma among Caucasian people, followed by African descendents, and it is rarely detected in Asian population. According to Ratnesar7, this prevalence is very low in Inuit Eskimos, suggesting that their anatomical morphological characteristics may facilitate the aeration of the middle ear and prevent sequels of chronic otitis.
In the Ambulatory of Chronic Otitis Media, Hospital de Clínicas de Porto Alegre (AOMC-HCPA), out of 450 patients followed up since August 2000, 30% had cholesteatomatous chronic otitis media (CCOM), presenting bilaterally in 12% of the total sample. Out of the patients with CCOM, 45% were aged up to 18 years, considered to be pediatric patients. As to gender, we found 70% of male cases 8.
Classification
Cholesteatomas are normally classified as congenital and acquired and they are subdivided into primary and secondary.
Congenital cholesteatomas are epithelial remains found in ears with intact tympanic membrane and without previous history of infections 9. According to Valvassori10, they are found in four regions of the temporal bone: tympanic-mastoid, petrous apex, cerebello-pontine angle and jugular foramen. There is a fifth explanation, described by Sobol11, that reported the existence of small epithelial pearls between the tympanic membrane layers.
Acquired primary cholesteatomas are resultant from tympanic retractions that would accumulate the desquamated epithelium and would lose their self-cleaning power. Secondary cholesteatomas would be formed from the migration of the epithelium through a marginal perforation in the tympanic membrane (Costa et al., 1999).
Meyerhoff and Truelson6 tried to classify cholesteatomas according to the pathophysiology, location, ossicle defects and presence of complications, dividing them into congenital and acquired, and the latter into primary, secondary and tertiary.
Tos6 proposed another classification based on site of origin of cholesteatoma, which considers it as an important factor for the surgical procedure and the prognosis. This taxonomy presents three categories:
1. Attic Cholesteatoma - retraction of the flaccid pars or Shrapnell membrane, extending to the attic, going through the aditus and eventually reaching the antrum, mastoid or tympanic cavity.
2. Tympanic sinus Cholesteatoma - posterior-superior retraction or perforation of pars tense, extending to the tympanic sinus and posterior portion of the tympanic membrane.
3. Pars tense Cholesteatoma - retraction and total adhesion of the pars tense of tympanic membrane involving the tympanic orifice of the auditory tube.
Saleh and Mills13 proposed another classification, according to the site affected by the cholesteatoma, characterized as follows:
S1 - if the cholesteatoma is restricted to the site where it had started;
S2 - when the disease extends to other site;
S3 - if it affects three sites;
S4 - if it is installed in four sites;
S5 - to cases in which the primary site is affected plus four or more are also involved.
The same authors distinguished seven sites used to this classification: attic and antrum, middle ear, mastoid, auditory tube, labyrinth and middle fossa. The staging is a practical solution to describe disease extension and it is clinically relevant, which may be applied to all lesions except to the petrous apex, which cannot be diagnosed by otoscopy.
Saleh and Mills13 also presented a classification of the ossicle chain condition, based on the descriptions by Wullstein (1956) and Austin (1969), through the following score:
0 - if the ossicle chain is intact;
1 - if incus is eroded and without chain discontinuity;
2 - if incus and stapes suprastructures are eroded;
3 - if the malleus head and incus are absent and stapes superstructure if eroded.
As to preoperative complications, Saleh and Mills13 classified cholesteatomatous chronic otitis media as:
C0 - when there are complications;
C1 - to the occurrence of complications;
C2 - to the existence of two or more.
As to complications, the authors consider lateral semicircular canal fistula, facial palsy, total sensorineural auditory loss, sinus thrombosis and intracranial invasion.
Deep understanding about the pathogenesis of the middle ear cholesteatoma is particularly important, given that the destructive nature is responsible for many of the referred complications. The trend to bone erosion of cholesteatomas and the lack of non-surgical treatment justify the importance of the investigation of basic mechanisms related to development of cholesteatomatous chronic otitis media.
Etiopathogenesis
Ferlito14 described that three disposing conditions would be necessary for the development of cholesteatoma: a) the junction of two different epithelia in the auditory opening; b) the chronic destruction of the middle ear submucous layer by infectious and inflammatory processes; c) the scaring process or the proliferation phase.
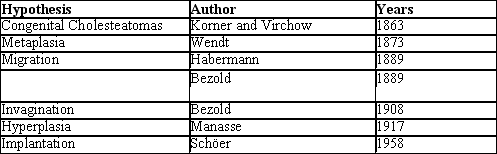
However, the etiopathogenesis of cholesteatomas is still very much discussed and basically there are six main hypotheses (Chart 1), which have generated controversy for over 100 years.
There are a number of studies about the pathogenesis of cholesteatomas, but there is still a lot to be clarified 15. As observed in AOMC-HCPA and in many different studies of this group 8,16-22 these hypotheses would not individually explain the pathogeneses of all cholesteatomas.
The existence of congenital cholesteatomas and the onset of cholesteatoma by invagination and implantation is unequivocal, but these situations would not be responsible for all cases of CCOM. We believe that the pathogenesis of cholesteatoma in fact would involve many different aggregated hypotheses (Chart 1), and there may be an overlapping of two or more of them in each patient.
As a consequence of this fact, it seems that the hypothesis of the continuum, advocated by Michael Paparella23, in 1970, is close to a multifactorial and more comprehensive conception of pathogenesis. According to it, otitis media would exist throughout a continuous series of epithelial and subepithelial events, in which after the initial insult, the serous or purulent otitis would become serum-mucoid, mucoid or finally, if there were no spontaneous or therapeutic regression of the condition, there would be chronic transformation. We can say, based on this hypothesis, that the cholesteatoma is a complex of one single middle ear pathology, chronic otitis media, and not an isolated event.
Structure
Macroscopically, cholesteatoma is a round or oval cystic lesion with variable configuration and size. Ferlito et al.14 characterized cholesteatoma as an epidermoid cyst of independent and progressive growth with destruction of adjacent tissues, especially the bone tissue, with tendency to recurrence.
The advent of transmission electron microscopy allowed many advances in the knowledge about cell structure. Using this instrument, Lim and Saunders24 in 1972 presented a detailed histological description of cholesteatomas. They described that the cholesteatoma has a keratinized stratified squamous epithelium with four layers identical to normal epidermis (basal, spinosum, granulosum and corneal), Langerhans cells (in larger amounts than in normal epidermis) and keratin-hyaline granules. They named this epithelium as cholesteatoma matrix. They also observed the presence of connective tissue, containing collagen fibers, fibrocytes and inflammatory cells, which was named perimatrix, which is in most of the cases in contact with squamous or ciliated cylindrical cells, remains from the original middle ear mucosa. In some cases, despite the fact that the perimatrix is absent at optical microscopy, it was present when studied under transmission electron microscopy, proving to be extremely thin, with collagen fibers that were practically absent and containing sodium carbonate crystals. In the study conducted by Paludetti et al.25, the perimatrix consisted of granulation tissue or inflamed subepithelial connective tissue.
Chart 1. Main hypotheses for the etiopathogenesis of cholesteatomas.
According to Milewski et al.26, the growth of cholesteatoma could require angiogenesis in the perimatrix connective tissue, and cells and substances of the scaring cascade would have an important role in the development and growth of cholesteatoma. These processes would involve the fibroblastic growth factor b (b-FGF), which according to some authors, could stimulate collagenase. They also suggested that persistence of inflammation would cause a permanent process of scaring in the perimatrix, proliferation of fibroblasts (granulation tissue) and epithelium (matrix).
Ferlito et al.14 described the perimatrix as the most peripheral portion of the cholesteatoma, comprising granulation tissue or inflammatory subepithelial connective tissue, with lymphocytes, hystiocytes and neutrophils. Sprekelsen et al.27 stated that the matrix and perimatrix, in normal or pathological tissues, are formed by type IV collagen, tenascin, fibronectin, b-FGF and metalloproteinase (MMP). According to Jacob et al.28, the increment in proliferation of the cholesteatoma matrix would be the result of the inflammation process, suggesting that the perimatrix would be the main factor of cholesteatoma development.
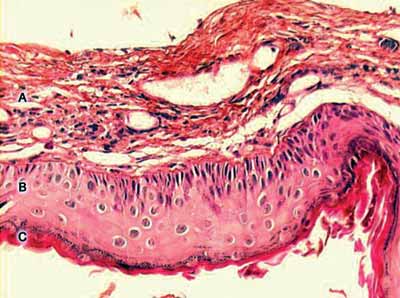
In summary, we can define the perimatrix as an inflammatory network that involves the cholesteatoma. Figure 1 presents a histological section of a cholesteatoma, in which we can see the structural components: perimatrix, matrix and cystic content.
Pereira et al.29 studying 31 cholesteatomas - 20 adults and 11 children, found nine specimens without perimatrix, visible under optical microscopy, two in adults and seven in pediatric cases. These findings corroborate those reported by Lim and Saunders24, which confirmed that many perimatrixes can only be seen under electron microscopy. These authors suggest that a possible cause of this reduction in collagen fibers could be the result of a differential action of collagenase.
Hamsei et al.30 analyzed 21 cholesteatomas through polymerase chain reaction (PCR), immunohistochemistry and histology, with the objective of investigating stimulation factors and differentiation of osteoclasts into cholesteatoma, using skin from the external acoustic canal as control. The immunohistochemical analysis demonstrated an increase in osteoclast and macrophage precursor cells in cholesteatomas. The perimatrix analysis showed that in these regions of the cholesteatoma, there are all factors necessary for osteoclastogenesis and for stimulation of bone reabsorption.
Biology of Cholesteatoma
The study of cells under optic and electron microscopy may give us the mistaken impression that they are static structures. However, to the contrary, many processes and movements are constantly happening inside the cells, occurring in some tissues quicker than in others. It is easy to understand that as cells are getting differentiated, and parallel they acquire some structural and physiological particularities.
Epithelia are tissues with limited life, with constant renewal, resulting from continuous mitotic activity. The speed of cell replacement is variable, and it may range from 2 to 50 days, depending on the tissue to be considered 31.
The connective tissue, in turn, comprising cholesteatoma perimatrix, presents a growth process that is more complex because it is formed by different types of cells - fibroblasts, macrophages, mast cells, plasmocytes, leukocytes - separated by abundant intercellular material. Richness of this material is one of its most important characteristics. It is a part with defined microscopic structure - connective fibers - and by another non-structured portion - a substance that is fundamentally amorphous 31. Owing to this complexity, one could expect that the renewal process of this tissue would be quite complex.
Figure 1. Digitalized image of the lamina, cross section of a cholesteatoma, stained with Hematoxylin-Eosin. We can see three forming parts: A - perimatrix, B - matrix, C - cystic content.
Cholesteatomatous chronic otitis media could be the result of an uncontrolled cell proliferation; more simply, in this pathology there is growth of keratinized squamous epithelium cyst, the cholesteatoma14. It could be considered as a disorder of the control of cell growth, comprising a series of complex and dynamic events involving cell and extracellular components with affections to their biological behavior, such as keratinocyte deregulation 32, which present a hyperproliferative growth and cell differentiation affections. However, it is not known for sure whether this lack of control is caused by defect to the genes that control the proliferation, by cytokines released by inflammatory cells or by mechanisms still unknown 12. Thus, determining the existence of defects in its biology, biochemistry and genetics is essential to learn about the pathogenesis.
As previously described, the capacity to invade, migrate, modify the differentiation, proliferation and recurrence of cholesteatomas is quite similar to neoplasms, but there is reluctance between researchers about accepting the inclusion of cholesteatoma in this category 33,34.
For cholesteatoma to be considered a neoplastic lesion it is necessary to have evidence of genetic instability; it may be manifested through DNA affection or specific chromosomic abnormalities. In 1995, Shinoda and Huang35 detected the protein p53 in cholesteatomas, suggesting that they could be tumors. However, Desloge et al.33 demonstrated that there were no DNA affections, ruling out this hypothesis.
Given that the reported studies have not detected any genetic instability in these lesions, we should investigate another possible reason for the development of CCOM, being necessary to ask about the origin of keratinized squamous epithelium in cholesteatomas. To study this issue, many investigations using immunohistochemical analysis have been performed to compare the location of differentiation markers in cholesteatomas and on the skin of the external auditory canal. Owing to the properties of cytokeratins, they have been considered by many investigators 36,37,32 as the best instruments to this end.
Cytokeratins are proteins that form one of the two categories of intermediate filaments, located on the cytoplasm of epithelial cells; they have 20 subclasses, and their expression is dependent on the type of epithelium and stage of differentiation38. Pereira39, Albino et al.40 and Kim and Chung41 reported that matrix of cholesteatomas expresses cytokeratin 16 (CK16) on suprabasal layers, being that the expression of this protein filament is characterized by hyperproliferative epithelium. Lepercque et al.42 described that CK16 does not show on the normal epithelium unless it is in areas under pressure and friction, or on the recover epithelium of hair follicles. According to Broekaert et al.43, CK16 is expressed in specific regions, such as tympanic ring and medial and inferior regions of the external acoustic canal. Pereira et al.29 stated that the presence of CK16 in the matrix of the cholesteatoma could indicate a hyperproliferative behavior, similar to hyperproliferative epidermal disease. According to Albino et al.40, cholesteatoma is formed as a result of the attempt to repair the lesion, which could explain the presence of CK16, characterizing this epithelium as immature with predominance of cell proliferation. Kujipers et al.37 analyzed the pattern of cytokeratins and suggested that the cholesteatoma matrix is not a result of a metaplastic change. In the study, they found an epithelium similar to that of the tympanic membrane and to the skin of the external auditory canal, but in different stages of proliferation, depending on the level of inflammation present.
A characteristic signal of cholesteatoma is the infiltration in the perimatrix of immune system cells. Piltcher44, in his thesis about cytokines in chronic otitis media with effusion, stated that in addition to the known risk factors, such as auditory tube dysfunction and infections, many research studies about otitis media have been directed to the study of different components of the inflammatory response. The essential issue is whether the inflammation should be considered only a process of defense or whether it has a role in perpetuation of cholesteatomatous chronic otitis media. Milewski45 suggested that inflammatory cytokines, fibroblasts and macrophages would be responsible for the origin, growth and bone destruction of cholesteatoma. Many cytokines and growth factors could be involved in the proliferation mechanism and development of cholesteatoma epithelium 46,47. Tomita48 stated that there are many hypotheses that growth factors and cytokines, present in cholesteatomas, induce the activation of genes, such as c-myc, causing the deregulation of cell proliferation. Sudhoff et al. 49 investigated the distribution and expression of tumor growth factor (TGF-alpha), epithelial growth factor (EGF-R) and oncogene c-myc in normal epithelial cells of middle ear and in cholesteatomas. These factors were found in the cholesteatoma matrix, but not in normal cells. In addition to autocrine regulation of epithelium, owing to production of epithelial growth factor (EGF), cholesteatoma hyperproliferation could depend on the interaction of the subepithelial tissue and the inflammatory changes that occurred in this pathology. Sudhoff et al.50 investigated the expression and location of growth factors of angiogenesis in 22 cholesteatomas in comparison to normal epidermis of external auditory canal (8) and to mucosa of normal middle ear (5), to identify some growth factors involved in the pathogenesis of cholesteatomas. These researchers found 5.3±1.2 vessels/mm2 on the normal skin and mucosa, whereas in the group with cholesteatoma there was 21.1±11.7 vessels/mm2, and the mean ranged according to degree of inflammation of perimatrix: 9.0±3.5 vessels/mm2 in grade I, 19.2±3.6 vessels/mm2 in grade II and 31.7±9.4 vessels/mm2 in grade III. They also reported a reduction in collagen type IV and laminin on the basal membrane of the cholesteatoma, comparing to the controls.
A common characteristic in the pathogenesis of many types of cholesteatoma is the presence of bacteria, consequently, a large amount of cytokines released by inflammatory cells resulting from the immune response. The presence of bacteria to promote a critical link between cholesteatoma and the host, preventing the neoformed epithelium to conclude its process of differentiation, which would promote a quiescent status, minimally proliferative, without migration or invasion in this step 51. The interactions between inflammatory cells and epithelium of the cholesteatoma could be responsible for the induction of aberrant biological characteristics of the pathology.
Chole and Faddis51 studied, under electron transmission microscopy, 24 human cholesteatomas and 22 cholesteatomas in Mongolian squirrels. In the human sample, 16 presented histological findings consistent with biofilm bacteria, whereas the material from the animal study showed 21 evidences of bacteria. These findings could be related with the activity of cholesteatoma, especially in persistent or recurrent infections and with resistance of topical or systemic antimicrobials. The authors suggested that the cholesteatoma matrix is an ideal medium for the development of a mixed microbiological biofilm. The authors stated that even though the bacteria in the biofilm are resistant to antibiotics by different mechanisms used by plankton bacterial, the exact mechanism of resistance to bacteria colony in biofilm is still unknown.
Studies published so far presented many data about the biology of cholesteatomas, but many questions still remain. As previously referred, cholesteatomas present neoplastic characteristics (invasion, migration, change in differentiation), but to present, no indication of genetic instability has been detected in the structure, a fact that rules out the possibility of considering it a neoplasm. Another property that seems to be constant in cholesteatomas is its hyperproliferative activity, which might be a possible explanation to its aggressive characteristics and uncontrolled growth. In addition, stimuli of immune response, represented by cytokines related to perimatrix inflammatory cells, represent a strong candidate to the role of key player in this intricate plot of mechanisms. All these hypotheses make us consider the complexity involved in the biology of cholesteatomas and consequently the multitude of events related with its pathogenesis.
Power of Bone Erosion
Cholesteatomas have great power of bone erosion 52,53. They normally reach the ossicle chain and less frequently the head bones, including the most rigid bone of the human body, the labyrinth, which demonstrates its strong destructive action over the bone tissue. Partial or total destruction of ossicle chain is observed in about 80% of the patients with cholesteatoma, whereas in non-cholesteatomatous chronic OM there is erosion of ossicle chain in approximately 20% of the cases 54,55. The mechanisms that lead to this increase in bone degradation and invasion are currently being investigated 16.
According to Fisch56, complications caused by cholesteatomas may be divided into two groups: intracranial ones - meningitis, abscesses and venous sinus thrombosis, and temporal bone ones - mastoiditis, labyrinthic fistula, facial nerve palsy, labyrinthitis and ossicle destruction.
Sadé and Berco57 performed a histological exam in 80 ossicles obtained in surgery and 41 of them were from patients with NCCOM and 39 from CCOM. Bone erosion was found in 42.5% of the ossicles of patients without cholesteatoma and in 84% of the patients with cholesteatoma, and the difference was statistically significant (P<0.001). In the study by Prescott58, 81 children with CCOM were followed up; out of a total of 96 cholesteatomas (15 bilateral), there were only 19 integral ossicle chains, 23 presented erosion of malleus and intact suprastructure, 51 had erosion of malleus and loss of suprastructure, and three were not described.
Sadé and Fuchs59 compared the findings of ossicle erosion in adults (age ???? years) to those found in children (age ? 13 years). The percentage of destruction of stapes and malleus were similar in both groups; incus presented significantly greater destruction in adults. Facial nerve palsy and labyrinthic fistula were equally more destroyed in adults. Dornelles et al.16 performed a study of the description of middle ear findings in the transoperative period of 55 patients with chronic otitis media, followed in the AOMC-HCPA. Out of these patients, 49% had diagnosis of CCOM. In the sample, there were 66% of cases with involvement of ossicle chain: in cases of CCOM the rate was 96% whereas in NCCOM it dropped to 37%. The presence of cholesteatoma was associated with existence of two or more affected ossicles, as well as higher prevalence of absence or erosion of ossicles. These findings indicate that most patients with COM, submitted to surgical intervention, have ossicle chain involvement and that frequency and extension of impairment were much more related with presence of cholesteatoma. In our group, we categorized ages as: children up to 18 years and adults as of 19 years. When we analyzed the ossicle chain, observed in the transoperative analysis of patients with CCOM, we found 100% of impairment in children and 92% in adults. When we compared the ossicles separately, we reached 30% of affections in the malleus, 30% in the stapes and 90% in the incus, without statistically significant difference between adults and children. Upon repeating the analysis following the same age classification used by Sadé and Fuchs59, the results were different from the ones reached by the authors, because we did not find statistically significant differences when comparing ossicles in children and adults 16.
According to Swartz60, ossicle destruction is more common among complications of cholesteatomas and the type of destruction depends on origin and mode of expansion. According to the data, the ossicle chain is intact in only 26% of the attic cholesteatomas, and the long process of incus is the most affected region, followed by incus body and malleus head. In turn, pars tense cholesteatomas have power of erosion of 90%.
Bone absorption is stimulated by a variety of factors, including inflammation, local pressure, specific cytokeratins and keratin 6. The enzymatic concept in which enzymes of epithelial origin are considered responsible for bone destruction was defined by Abramson61-64, which demonstrated the presence of collagenase and hydrolase in cholesteatoma, a hypothesis that was later confirmed by Thompsen65. Ferlito et al.14 suggested that the destructive property of cholesteatomas - the bone erosion is caused by the production of collagenase by the components of squamous and fibrous epithelial tissues. It is not properly demonstrated yet whether mineralized bone can be absorbed by collagenase. Other agents were incorporated, such as tumor necrosis factor (TNF), interleukins (IL-1a) and prostaglandins (PGE2)66-68, to the hypothesis of bone absorption by biochemical action, exclusively played by collagenolytic enzymes.
Peek et al.69 studied the concentration of lipopolysaccharides, components of the membrane of gram-negative bacteria 70 in cholesteatomas and compared to the level found in samples of patients with NCCOM. They found higher concentrations in patients with cholesteatoma and suggested that these results would be related with high levels of bone reabsorption.
Pediatric Cholesteatoma
There are some references supporting that cholesteatomas are more aggressive and would have less favorable prognosis in children than in adults 71-75. Smythe et al.76 consider that clinical behavior of pediatric cholesteatoma is so different in adults that it should be considered a different disease.
Quaranta et al.77 tried to check whether the clinical behavior of cholesteatomas in children would depend on histomorphological characteristics of the perimatrix. They compared the number of plasmocytes, lymphocytes, macrophages, granulocytes and giant cell in the perimatrix of samples taken from 30 patients aged less than 16 years with those of 30 adults, used as controls. The results suggested that in children the number of inflammatory mononuclear elements of the perimatrix would be higher than in adults, with evident activity of the collagenase enzyme. Based on this behavior, the authors suggested that the perimatrix characteristics could place an important role in the pathogenesis of cholesteatoma, and it could be one of the factors that justified the clinical differences between children and adults.
Bujia et al.73 analyzed the expression of MIB1 (monoclonal antibody marker of cell proliferation) in 20 cholesteatoma in children, whose controls were 15 cholesteatomas in adults and skin of the external auditory canal. Immunohistochemical analysis showed normal indexes of epidermis in the canal and increased levels in both categories of cholesteatomas. However, the number of cells in proliferation was significantly higher in the pediatric group (p<0.01). When they compared infected cholesteatomas with non-infected ones, we did not find any significant difference, suggesting that the index of proliferation could be independent from external factors.
Conversely, Edelstein78 stated that pediatric cholesteatoma would be less expansive, which would take to smaller incidence of complications and that adults would be more susceptible to facial palsy, intracranial infection, labyrinthic fistula and ossicle erosion. Disagreeing with this author, Darrouzet et al.79 suggested that fewer complications in the pediatric group would be caused by the fact that in this group the duration of the disease is on average below what is found in adults.
However, the consensus among most of the authors is that recurrent cholesteatoma, that is, development of a new cholesteatoma after surgical treatment, and residual cholesteatoma, which is originated from the growth of non-removed parts of the cholesteatoma during the surgery 80, are more common in the pediatric group and the average recurrence rate is 30%, compared to 3% to 15% in adults 78,81. Prescott58 followed 81 children with CCOM in a cohort of 10 years and found a percentage of cholesteatoma recurrence of 12%. Lino et al.82 conducted a study in 83 children with CCOM and found 25% of recurrence, whereas residual cholesteatoma was diagnosed in 42%. These authors suggested that recurrence of cholesteatoma could be intimately related with auditory tube dysfunction and that high rate of proliferation of matrix could be responsible for residual cholesteatomas.
Ruah et al.72 suggested that persistence of mesenchymal and major inflammatory reaction observed in the flaccid pars and in the posterior-superior quadrant of pars tense of tympanic membrane, as well as collagen and elastic affections observed in purulent and serous otitis media, can represent a pathological justification for the typical development of cholesteatoma in children.
Management of Cholesteatoma
Treatment of CCOM is essentially surgical. The primary objective is complete eradication of the disease, providing to the patient a dry and complication-free ear. The secondary objective, although not less important, is to preserve or improve the tympano-ossicle system function, whenever possible 83.
The first one is reached through meticulous removal of the whole cholesteatoma (including the matrix and perimatrix in the wall up technique) and other compromised tissues. To that end, a variety of surgical techniques has been used, but they are summed up into two basic ones, depending on the removal or sparing of the posterior wall of the external auditory canal: wall up or wall down mastoidectomy. Regardless of the technique, the secret to surgical success is complete eradication of the disease. The selection of the procedures is based on type, grade and extension of cholesteatoma; in the preoperative auditory assessment, including presence of associated complications, contralateral ear status, together with auditory tube function and level of mastoid pneumatization. This choice will also depend on general patients\' conditions, age, origin and profession 1.
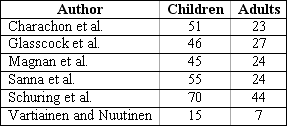
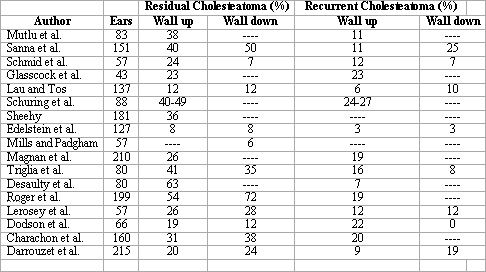
The wall down technique can be safer concerning eradication and prevention of recurrence, but it does not allow preservation of the anatomy and sometimes the level of preoperative hearing 84. We should bear in mind that the approach creates a cavity that will require careful and long medical follow up in addition to demanding general care for the whole life of the patient, characterizing a limiting factor for some sports such as swimming and scuba diving 85. However, the wall down technique, when compared to the wall up technique, presents less incidence of residual cholesteatoma 86, regardless of the fact that the rate varies a lot depending on the author 87-92. In all studies, it is always higher among children (Charts 2 and 3, below, adapted from Darrouzet et al.79).
Chart 2. Incidence of residual and recurrent cholesteatomas, stratified by age group, as presented in the literature.
Chart 3. Frequency of residual and recurrent cholesteatomas, stratified by surgical technique as presented in the literature.
Since the first report of mastoidectomy technique, in 1649, by Riolanus, up to today, the surgery has been through a gradual evolution, supported by field studies and technological development. However, surgical management of cholesteatomas in children is still the key controversy among otologists 93-95. To define the best therapeutic strategy, especially in this special group of patients, it is necessary to consider many factors such as total eradication of the disease and preservation of auditory function; for the final decision, all factors should be properly considered and analyzed. The key issue is that up to present, no histological or biochemical differences have been found among cholesteatomas in different age ranges that could confirm or not the aggressiveness of CCOM in pediatric cases, a fact that would justify the use of less conservative surgical techniques.
The next steps
One of the first steps in the advance of understanding and treating cholesteatomas is the interaction between clinical, histological and experimental studies.
The connection between these three lines of research will be essential to understand CCOM.
REFERENCES
1. Cruz OL, Costa SS. Otologia Clínica e Cirúrgica. Ed. Revinter; 1999.
2. Costa SS, Roithmann R, Matheus MC. A patogênese dos colesteatomas. Rev Bras Otor 1992; 57(4): 30-5.
3. Friedmann I. Epidermoid cholesteatoma and granuloma. Ann Otol Rhin Laryngol 1959; 68: 57-9.
4. Schuknecht HF. The pathology of the ear. Cambridge: Harvard University; 1974.
5. Tos M. Recurrence and the condition of the cavity after surgery for cholesteatoma using various techniques. Kugler and Ghedini; 1989.
6. Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, Sudhoff H. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol 2004; 261(1): 6-24.
7. Ratnesar P. Aeration: a factor in the sequels of chronic ear disease along the Labrador and northern Newfoundland Coast 1977.
8. Dornelles C, Hemb L, Schweiger C, Matter R, Smith M, Schimdt L, Costa SS. Epidemiologia dos pacientes pediátricos do ambulatório de otite média crônica no Hospital de Clínicas de Porto Alegre (AOMC-HCPA). Rev Bras de Otorringol 2004. No prelo.
9. Derlacki EL, Clemis JD. Congenital cholesteatoma of the middle ear and mastoid. Ann Otol Rhinol Laryngol 1965; 74: 706-27. In: Cruz OL, Costa SS. Otologia Clínica e Cirúrgica. Ed. Revinter; 1999.
10. Valvassori GE. Benign tumors of the temporal bone. Radiol Clin North Am 1974; 12: 533-42. In: Cruz OL, Costa SS. Otologia clínica e cirúrgica. Ed. Revinter; 1999.
11. Sobol SM. Intramembranous and mesotympanic cholesteatomas associated with an intact tympanic membrane in children. Ann Otol 1980 98: 312-17. In: Cruz OL, Costa SS. Otologia Clínica e Cirúrgica. Ed. Revinter; 1999.
12. Costa SS, Hueb MM, Ruschel C. Otite média crônica colesteatomatosa. In: Cruz OL, Costa SS. Otologia Clínica e Cirúrgica. Ed. Revinter; 1999.
13. Saleh HA, Mills RP. Classification and staging of cholesteatoma. Clin Otolaryngol 1999; 24: 355-9.
14. Ferlito O, Devaney KO, Rinaldo A, Milroy C, Wenig B, Iurato S, McCabe B.F. Clinicopathological consultation ear cholesteatoma versus cholesterol granuloma. Ann Otol Rhinol Laryngol 1997; 106: 79-85.
15. Sculerati N, Bluestone C. Pathogenesis of cholesteatoma. The Otol Clin of North Am 1989; 22 (5): 859-68.
16. Dornelles C, Weber R, Schimdt VB, Schimdt L, Dall´Igna D, Carvalhal LHSK, Kruse L, Costa SS. Descrição da cadeia ossicular no trans-operatório de pacientes com otite média crônica. Pesquisa: Logos e Práxis. Unidade de Pesquisa do Instituto de Cardiologia; 2002. p. 221.
17. Dornelles C, Costa SS, Laux M, Weber R. Estudo comparativo da dissolução de três diferentes marcas de colágeno utilizadas em técnicas cirúrgicas otológicas. Rev Bras de Otorrinolaringol 2003; 69(6): 744-51.
18. Hemb L, Schweiger C, Matter R, Dornelles C, Smith M, Schmidt L, Costa SS. Papel da via de formação do colesteatoma na orelha contralateral em pacientes pediátricos do AOMC-HCPA. Revista On-Line do HCPA www.hcpa.ufrgs.br 2003.
19. Schweiger C, Hemb L, Matter R, Schmidt L, Smith M, Dornelles C, Costa SS. Perda auditiva sensorioneural em crianças com otite média crônica. Revista on line do HCPA. www.hcpa.ufrgs.br 2003.
20. Matter R, Schweiger C, Hemb L, Smith M, Schmidt L, Dornelles C, Costa SS. Estudo da orelha contralateral na otite média crônica em pacientes pediátricos. Revista do HCPA www.hcpa.ufrgs.br 2003.
21. Schmidt V, Dornelles C, Weber R, Schimdt L, Dall´Igna D, Carvalhal L, Kruse L, Costa SS. Perda auditiva condutiva na otite média crônica com perfuração central ou marginal. Pesquisa: Logos e Praxis. Unidade de Pesquisa do Instituto de Cardiologia; 2002 p. 220.
22. Scheibe AB, Smith MM, Schmidt LP, Schmidt VB, Dornelles C, Carvalhal LHSK, Kruse L, Costa SS. Estudo da orelha contralateral na otite média crônica: "Efeito Orloff". Rev Bras Otorrinolaringol 2002; 68(2): 245-9.
23. Paparella MM, Hiraide F, Juhn SK, Kaneco J. Cellular events involved in middle ear fluid production. Ann Rhinol Otol Laryngol 1970; 79(4): 766-79. VER.
24. Lim DJE, Saunders WE. Acquired cholesteatoma: light and electron microscopic observations. Ann Otol 1972; 81: 2-12.
25. Paludetti G, Alamadori G, Ottaviani F, Rosignoli M, Rossodivita M, D´Alatri L. Ultrastructural aspects of cholesteatoma of the middle ear. Acta Otorhinolaryngol Ital 1989; 9(2): 169-80.
26. Milewski C, Fedorowski A, Stan AC, Walter GF. Basic fibroblast growth factor (b-FGF) in the perimatrix of cholesteatoma. HNO 1998(a) 46(9): 804-8.
27. Sprekelsen BM, Ebmeyer J, Anonopoulos A, Sudhoff H. Alteraciones de la membrana basal en el colesteatoma de oído medio. Acta Otor Esp 2001; 52: 330-5.
28. Jacob R, Welkoborsky HJ, Mann W. Epithelium-stroma interaction in cholesteatoma of the middle ear. Laryngorhinootologie 2001; 80 (1): 11-7.
29. Pereira CSB, Almeida CIR, Vianna MR. Imunoexpressão da citoqueratina 16 e do antígeno nuclear Ki-67 no colesteatoma adquirido da orelha média. Rev Bras de Otorrinolaringologia 2002; 68(4): 453-60.
30. Hamzei M, Ventriglia G, Hagnia M, Antonopolous A, Bernal-Sprekelson M. Dazert S, Hildmann H, Sudhoff H. Osteoclast stimulating and differentiating factors in human cholesteatoma. Laryngoscope 2003; 113(3): 436-42.
31. Junqueira LCE, Carneiro J. Histologia Básica. Rio de Janeiro: Editora Guanabara; 1985.
32. Vennix PP, Kuijpers W, Peters TA, Tonnaer EL, Ramaekers FC. Keratinocyte differentiation in acquired cholesteatoma and perforated tympanic membranes. Arch Otolaryngol Head Neck Surg 1996; 122: 825-32.
33. Desloge RB, Carew JF, Finstad CL, Steiner MG, Sassoon J, Levenson MJ, Staiano-Coico L, Parisier SC, Albino AP. DNA analysis of human cholesteatomas. Am J Otol 1997; 18(2): 155-9.
34. Albino AP, Reed JA, Bogdany JK, Sassoon J, Desloge RB, Parisier SC. Expression of p53 protein in human middle ear cholesteatomas: pathogenetic implications. Am J Otol 1998(a); 19(1): 30-6.
35. Shinoda H, Huang CC. Expressions of c-jun and p53 proteins in human middle ear cholesteatoma: relationship to keratinocyte proliferation differentiation and programmed cell death. Laryngoscope 1995; 105(11): 1232-7.
36. Bujia J, Schiling V, Holly A, Stammberger M, Kastenbauer E. Hyperproliferation-associated keratin expression in human middle ear cholesteatoma. Acta Otolaryngol 1993; 113: 364-8.
37. Kuijpers W, Vernnix PP, Peters TA, Ramaekers FC. Squamous metaplasia of the middle ear epithelium. Acta Otolaryngol 1996; 116: 293-8.
38. Moll R, Frankev W, Schiller DL. The catalog of human cytokeratins: patterns of expression in normal epithelia tumors and cultured cells. Cell 1982; 31: 11-24.
39. Pereira CSB. Análise de estudos da expressão das citoqueratinas no colesteatoma adquirido. Dissertação de Mestrado. Faculdade de Ciências Médicas da Santa Casa de São Paulo; 1997.
40. Albino AP, Kimmelman CP, Parisier SC. Cholesteatoma: a molecular and cellular puzzle. Am J Otol 1998(b); 19: 7-19.
41. Kim CS, Chung JW. Morphologic and biologic changes of experimentally induced cholesteatoma in Mongolian gerbils with anticytokeratin and lectin study. Am J Otol 1999; 20(1): 13-8.
42. Lepercque S, Broekaert D, Van Cowwenberge P. Cytokeratin expression patterns in the human tympanic membrane and external ear canal. Eur Arch Otorhinolaryngol 1993; 250: 78-81.
43. Broekaert D, Coucke P, Lepercque S, Ramaekers F, Van Muijen G, Boedts D, Leigh I, Lane B. Imunohistochemical analysis of the Cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann Otol Rhinol Laryngol 1992; 101: 931-8.
44. Piltcher O. Um novo modelo experimental para investigação da otite média com efusão e sua aplicação no estudo das citocinas durantes as diferentes fases dessa doença. Tese de Doutorado. Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2000.
45. Milewski C. Role of perimatrix fibroblast in development of acquired middle ear cholesteatoma. A hypothesis. HNO 1998(b); 46(5): 494-501.
46. Aumente PO, Bujia J, Kim C, Jimenez-Gimenez J, Lopez-Villarejo P. Estudio cuantitativo de la presencia de interleucina-1 e interleucina-6 en el colesteatoma de oído medio. Acta Otorrinolaringol Esp 1996; 47(4): 259-62.
47. Bujia J, Kim C, Ostos P, Kastenbauer E, Hultner L Role of interleukin 6 in epithelial hyper proliferation and bone resorption in middle ear cholesteatomas Eur Arch Otorhinolaryngol 1996(a); 253(3): 152-7.
48. Tomita S. Aspectos moleculares do colesteatoma - imunoexpressão das proteínas controladas do ciclo celular: p53 BAX e BCL-2. SP Tese de Doutorado. Escola Paulista de Medicina; 2000.
49. Sudhoff H, Borkowski G, Bujia J, Hildmann H. Immunhistochemische Untersuchungen Von Mittelohrschleimhautresten im Cholesteatom. HNO 1997; 45(8): 630-5.
50. Sudhoff H, Dazert S, Gonzales AM, Borkowski G, Park SY, Baird A, Hildmann H, Ryan AF. Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am J Otol 2000(b); 21: 793-8.
51. Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg 2002; 128: 1129-33.
52. Sadé J, Halevy A. The aetiology of bone destruction in chronic otitis media. J Laryngol Otol 1974; 88(2): 139-43.
53. Bretlau P, Jorgensen MB, Sorenses CH, Dabelsteen E. Bone resorption in human cholesteatomas. Ann Otol 1982; 91: 131-5.
54. Chole RA. The molecular biology of bone resorption due to chronic otitis media. Ann New York Acad Sci 1997; 830: 95-109.
55. Costa SS. Contribuição ao Estudo das Otites Médias Crônicas. Dissertação de Mestrado. Faculdade de Medicina de Ribeirão Preto, USP; 1991.
56. Fisch U. Intracranial complications of cholesteatoma. In: Swartz JD. Colesteatomas of the middle ear. Diagnosis etiology and complications. Radiol Clin North Am 1984; 22: 15-34.
57. Sadé J, Berco E. Bone destruction in chronic otitis media. A histopathological study. J Laryngol Otol 1974; 88(5): 413-22.
58. Prescott CAJ. Cholesteatoma in children - the experience at The Red Cross War Memorial Children\'s Hospital in South Africa 1988-1996. Int J Pediatric Otorhinolaryngol 1999; 49(1): 15-9.
59. Sadé J, Fuchs C. Cholesteatoma: ossicular destruction in adults and children. J Laryngol Otol 1994; 108(7): 541-4.
60. Swartz JD. Colesteatomas of the middle ear. Diagnosis Etiology and Complications. Radiol Clin North Am 1984; 22: 15-34.
61. Abramson M. Collagenolytic activity in middle ear cholesteatoma. Ann Otol 1969; 78: 112-25.
62. Abramson M. Collagenase in the mechanisms of action of cholesteatoma. Ann Otol Rhinol Laryngol 1971(a); 80(3): 414.
63. Abramson M, Gross J. Further studies on a collagenase in middle ear cholesteatoma. Ann Otol Rhinol Laryngol 1971(b); 80: 177-85.
64. Abramson M, Huang CC. Localization of collagenase in human middle ear cholesteatoma. Laryngoscope 1976; 86: 771-91.
65. Thompsen J. Bone resorption in chronic otitis media. In: McCabe BF, Sade J. Abramson M. eds. Cholesteatoma: first international conference. Birmingham Ala: Aescupulus Publishing Co.; 1977. p. 136.
66. Minotti AM, Kountakis SE, Leighton WR, Cabral FR. Effects of extracellular calcium on cholesteatoma migration and adhesion in vitro. Otolaryngol Head Neck Surg 1996; 115(5): 458-63.
67. Kurihara A, Toshima M, Yuasa R, Takasaka T. Bone destruction mechanisms in chronic otitis media with cholesteatoma: specific production by cholesteatoma tissue in culture of bone-resorbing activity attributable to interleukin-1 alpha. Ann Otol Rhinol Laryngol 1991; 100(12): 989-98.
68. Hansen T, Unger RE, Gaumann A, Hundorf I, Maurer J, Kirkpatrick J. Kriegsmann J. Expression of matrix-degrading cysteine proteinase cathepsin K in cholesteatoma. The United States and Canadian Academy of Pathology 2001; 14(12): 1226-31.
69. Peek FA, Huisman MA, Berckmans RJ, Sturk A, Van Loon J, Grote JJ. Lipopolysaccharide concentration and bone resorption in cholesteatoma. Otol Neurotol 2003; 249(5): 709-13.
70. Schorder NW, Heine H, Alexander C, Manukyan M, Eckert J, Hamann L. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J Immunol 2004; 173(4): 2683-91.
71. Glasscock ME, Dickins JFE, Wiet R. Cholesteatoma in children. Laryngoscope 1981; 91: 1743-53.
72. Ruah CB, Schachem PA, Paparella MM, Zelterman D. Mechanisms of retraction pocket formation in the pediatric tympanic membrane. Arch Otolaryngol Head Neck Surgery 1992; 118(12): 1298-305.
73. Bujia J, Holly A, Antoli-Candela F, Tapia MG Immunobiological peculiarities of cholesteatoma in children: quantification of epithelial proliferation by MIB1. Laryngoscope 1996(b); 106(7): 865-8.
74. Palva A, Karma P, Kärjä J. Cholesteatoma in children. Arch Otolaryngol 1997; 103(2): 74-7.
75. Sudhoff H, Tos M. Pathogenesis of attic cholesteatoma: clinical and imunohistochemical support for combination of retraction theory and proliferation theory. Am J Otol 2000(b); 21: 786-92.
76. Smythe JL, Brachman D, Grahm M. Complications of cholesteatoma: A report on 1.024 cases. In: Swartz JD. Colesteatomas of the middle ear. Diagnosis etiology and complications. Radiol Clin North Am 1984 22: 15-34.
77. Quaranta A, Ressa L, Santangelo A. Otomastoid cholesteatoma in children: histopathological findings. Int J Pediatric Otorhinolaryngol 1986; 12(2): 121-6.
78. Edelstein DR. Acquired cholesteatoma in pediatric age group. The Otoloraryngol Clin North Am 1989; 22(5): 955-64.
79. Darrouzet V, Duclos JY, Portmannd, Bebear JP. Preference for the closed technique in the management of cholesteatoma of the middle ear in children: a retrospective study of 215 consecutive patients treated over 10 years. Am J Otol 2000; 21(4): 474-81.
80. Caldas N, Caldas S. Considerações sobre colesteatomas residuais e iatrogênicos. Rev Bras Otor 1988; 54: 51-3.
81. Sien KCY. Cholesteatoma in children. Pediatric Clinics of North America 1996; 43(6): 1245-52.
82. Lino Y, Imamura Y, Kojima C, Takegoshi S, Suzuki JI. Risk factors for recurrent and residual cholesteatoma in children determined by second stage operation. Int J Pediatric Otorhinolaryngol 1998; 46(1-2): 57-65.
83. Costa SS, De Souza LCA, Andrade MI. Procedimentos sobre o temporal - revisando a nomenclatura. Rev Bras Otor 1991; 57(4): 170-9.
84. Hartwein J, Hormann K. A technique for the reconstruction of the posterior canal wall and mastoid obliteration in radical cavity surgery. Am J Otol 1990; 69(6): 333-6.
85. Ahn SH, Oh SH, Chang SO, Kim CS. Prognostic factors of recidivism in pediatric cholesteatoma surgery. Int J Pediatric Otorhinolaryngol 2003; 67(12): 1325-30.
86. Paparella MM, Moris MS, Costa SS. A one-stage compromise of the open vs. closed method. The IBM Procedure Proco of the Third Intem Conf on Cholesteatoma and Mastoid Surg. Amsterdam: Kluger Publications; 1988.
87. Sheehy JL, Brackmann DE, Graham MD. Cholesteatoma surgery: residual and recurrent disease. A review of 1.024 cases. Ann Otol Rhinol Laryngol 1977; 86(4): 451-62.
88. Shambaugh GE Jr. Endocrine aspects of Meniere's disease. Laryngoscope 1959; 69: 1027-32.
89. Brandow EC Jr. Surgical procedure for the mastoid cavity problem. Otolaryngol Head Neck Surg 1980; 88(5); 622-4.
90. Schuknecht HF. Myringoplasty. Clin Otolaryngol 1976; 1(1): 53-65.
91. Glasscock ME, Kanok MM. Tympanoplasty - a chronological history. Otolaryngol Clin North Am 1977; 10(3): 469-477.
92. Smyth GD. Postoperative cholesteatoma in combined approach tympanoplasty. Fifteen year report on tympanoplasty. Laryngol Otol 1976; 90(7): 597-621.
93. Edelstein DR, Parisier SC, Ahuja GS. Cholesteatoma in the pediatric age group. Ann Otol Rhinol Laryngol 1988; 97: 23-9.
94. Quaranta A, Cassano P, Carbonara G. Cholesteatoma surgery: open vs. closed tympanoplasty. Am J Otol 1988; 9(3): 229-31.
95. Mutlu C, Khashaba A, Saleh E. Surgical treatment of cholesteatoma in children. Otolaryngol Head Neck Surg 1993; 113: 56-60.
1 Master in Medical Sciences, Pediatrics, Ph.D. studies under course, Program of Post-graduation in Medical Sciences, Pediatrics - UFRGS, Biologist, Centro de Otite Média do Brasil.
2 Otologist, Ph.D. in Surgery, Joint Professor, Department of Ophthalmology and Otorhinolaryngology, Program of Post-graduation in Medical Sciences, Pediatrics and Post-graduation in Medical Sciences: Surgery, Federal University of Rio Grande do Sul.
3 Pathologist, Ph.D. in Sciences, Gastroenterology, Hired physician, Center of Pathology, Hospital de Clínicas de Porto Alegre; Advisor, Professor, Program of Post-graduation in Gastroenterological Sciences.
4 Resident physician, Hospital de Clínicas de Porto Alegre.
Service of Otorhinolaryngology - HCPA Service of Pathology - Hospital de Clínicas de Porto Alegre - HCPA.
Address correspondence to: Cristina Dornelles - Rua Cangussu 1343 Porto Alegre RS 90830-010.
Tel (55 51) 3249-2275 - E-mail: cristinadornelles@yahoo.com.br
This article was submitted through SGP, RBORL on 8/Mar/2005 and approved on 1/Apr/2005.