Year: 2002 Vol. 68 Ed. 1 - (12º)
Artigo Original
Pages: 69 to 73
Anatomical relation between the hypoglossal nerve and the carotid artery bifurcation
Author(s):
Felipe S. G. Fortes 1,
Dr. Erasmo S. Silva 2,
Dr. Luiz U. Sennes 3
Keywords: hypoglossal nerve, carotid artery, anatomy, endarterectomy, carotid body tumor.
Abstract:
Introduction: In the last decades the incidence of central nervous complications and death has decreased, especially in endarterectomy and carotid body tumor. Contrastingly, the incidence of cranial nerve following is a problem that remains high and little changed, and the hypoglossal nerve dysfunction is the most frequent. Aim: The aim of this study was to establish the anatomical relation between the carotid artery bifurcation and hypoglossal nerve. Study design: experimental. Material and method: Carotid artery and hypoglossal nerve dissections were carried out in 38 fresh corpses. All the individuals were placed in standard position and the dissections were performed with surgical technique. The measurements were done in centimeters and millimeters from the dissected carotid bifurcation to the XII nerve in the cervical area. Results: Twenty-six individuals were male and 12 female. The majority were whites, 30, and 8 were non-whites. The distance between hypoglossal nerve and carotid artery bifurcation ranged from 0.5 cm to 4.3 cm, with mean of 2.1 cm, median 2.0 cm and standard deviation of 0.63 cm. Neck length, age, gender and race were related with the measurements and failed to show significant statistic correlation (a > 0.05). Conclusion: In this sample there is a great anatomic variation of the distance between hypoglossal nerve and carotid artery bifurcation and there was no statistical difference concerning age, gender, race and neck length. A better understanding of the anatomic course of this nerve and its variation in relation to carotid artery bifurcation, are relevant to prevent hypoglossal nerve lesions in the carotid artery surgery.
![]()
1 Undergraduate, Medical School, USP.
2 Physician, Professor, Discipline of Human Structural Topography - Department of Surgery, Medical School, USP.
3 Ph.D., Professor, Discipline of Otorhinolaryngology, Medical School, USP. Director of the Service of Buccopharyngology, HCFMUSP.
Study presented at the 35º Congresso Brasileiro de Otorrinolaringologia - Special citation.
Address correspondence to: Felipe S. G. Fortes - R. Fernão Cardim, 159, ap. 154 Jd. Paulista - São Paulo - SP - 01403-020
Article submitted on September 19, 2001. Article accepted on October 05, 2001.
Introduction
Hypoglossal nerve damage is the most frequent complication after carotid artery surgery4, 24, especially endarterectomy12 and carotid body tumor5.
In the past decades, as a result of improvement on surgical techniques, complications such as mortality and central nervous system damage (cerebral vascular accident and transient ischemic episodes) after the carotid surgery reduced significantly2, 21. However, owing to the complexity of anatomic structures around the carotid bifurcation and the proximity with various cranial nerves, the incidence of cranial nerve dysfunction after surgery continues to be high, which has not changed much in past decades; moreover, the topic has not received enough attention in the literature1, 12, 23.
The incidence of cranial nerve lesion secondary to endarterectomy varies from 3 to 47.5% and the variation depends on type of study conducted (retrospective or prospective) and assessment method used2, 21. Studies presented different incidences of cranial nerve damage and the most frequently affected ones are hypoglossal nerve (1-17%)8, 23 and laryngeal recurrent nerve (1-8%)7, 19. Other prospect damaged nerves are mandible marginal nerve1, 12, 21, magnum auricular nerve1, 2, superior laryngeal nerve2, 7, accessory12, and more rarely, glossopharyngeal 6, transversal cervical22 and sympathetic trunk (Horner syndrome)14.
Similarly, the incidence of central neurological complications and mortality secondary to carotid body tumor decreased a lot and it is around 0 to 2%, but the lesion of cranial nerve is a problem that has not changed significantly in the past decades, amounting to a rate of 40%5, 17. Hypoglossal nerve is frequently the most affected one10, 16, 18, although some authors have considered the vagus as the most impaired nerve9, 11. Other possible damage may affect the nerves superior laryngeal5, 17, mandible5, 9 , 10, 18, pharyngeal branch of vagus5, glossopharyngeal5, 10, 11, 17, accessory5, 17, and sympathetic trunk5, 9 18.
The objective of the present anatomic study is to correlate hypoglossal nerve with the carotid bifurcation, determining the exact distance between the structures and to transform a clinical impression into objectively measured distances, in addition to calling the attention of surgeons to variability in the region.
material and Method
Between 1998 and 1999, we performed 38 dissections of carotid arteries in fresh cadavers submitted to necropsy, 26 males and 12 females, ages ranging from 18 to 85 years. There were 30 Caucasian and 8 non-Caucasian cadavers.
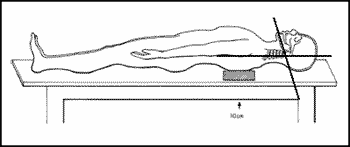
Dissections were performed in the Service of Death Investigation of the University of São Paulo. Cadavers were dissected in standard position, with a 10-cm pad on the basis of the scapula at 95o extension of the neck. Owing to the restrictions of the Service of Death Investigation concerning face and neck skin incisions, we performed an incision on the anterior thoracic wall. Next, the flap with skin and platysma muscle was mobilized superiorly up to the exposition of the carotid vessels, on the carotid triangle, parallel to the sternocleidomastoid muscle. Through surgical technique, the common carotid artery was isolated in its bifurcation. Dissection continued with the isolation of internal and external carotid arteries in their cervical portion. Hypoglossal nerve was identified at the point in which it crossed the carotid bifurcation and the relation was studied. The measure obtained was from the bifurcation up to the hypoglossal nerve, using a pachymeter, calibrated in millimeters. The length of the neck of the cadavers was measured from the mastoid process up to the jugular incision, so that it could be correlated with the studied distance.
Data were statistically analyzed with the software "Stat". The significance level was 5% (a=0.05%). Pearson's correlation method was used between variable pairs.
Results
The distance between the hypoglossal nerve and the carotid bifurcation varied from 0.5 to 4.3 cm (mean = 2.1 cm; median = 2.0 cm; standard deviation = 0.63 cm) in 38 studied subjects. There was no significant correlation between distance and age (r = -0.0786; p = 0.639), gender (r=-0.1519; p = 0.363), and race (r = -0.1951; p = 0.241).
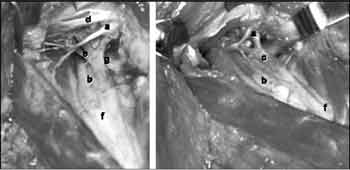
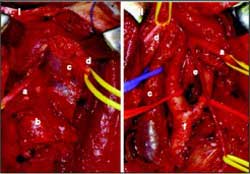
In 29 subjects, the neck length was correlated with the distance from the hypoglossal nerve and the carotid bifurcation, but there was no statistical significance (r = 0.1454; p = 0.452). The neck length varied from 14 to 29 cm (mean = 17.37 cm; median = 16.80 cm; standard deviation = 2.06 cm).Figure 1. Schematic representation of the position of the corpse for dissection (the angle between the horizontal plane and the mandible is 95º).Figure 3. Hypoglossal nerve and carotid bifurcation: a) hypoglossal nerve, b) internal carotid artery, c) descending branch of the hypoglossal nerve, d) digastric and stylohyoid muscles, e) occipital artery, f) carotid bifurcation, g) lingual artery.Figure 4. Carotid body tumor: before (I) and after surgical resection (II). a) hypoglossal nerve, b) carotid body tumor, c) internal jugular vein, d) accessory nerve, e) descending branch of hypoglossal nerve, f) carotid bifurcation.
Discussion
The hypoglossal nerve emerges from the brain through the hypoglossal canal, running posteriorly to the internal carotid artery and internal jugular vein by approximately 4cm. It crosses anteriorly the internal carotid artery, following inferior-laterally the internal carotid artery and the internal jugular vein, deeply into the posterior portion of the digastric muscle7, 8. Next, it crosses medially the internal and external carotid arteries over the carotid bifurcation, defining at this point a relation with the occipital artery (branch of the external carotid artery) and its sternocleidomastoid branch21. The descending branch of this nerve that originates from the hypoglossal nerve arch constitutes, together with the cervical nerves, the hypoglossal nerve loop8. Next, the hypoglossal nerve enters into the tongue base where it innervates the intrinsic and extrinsic tongue muscles. Hypoglossal nerve also innervates through the cervical loop the omohyoid, sternohyoid and sternothyroid muscles13.
The literature presents controversy about the distance between the hypoglossal nerve and the carotid bifurcation. According to most authors, the distance varies from 3.0 to 5.0cm20, 23, but there are descriptions of values of 1 to 2cm7.
Most atherosclerotic plaques of the carotid artery finish at about 2.0 to 3.0cm from the bifurcation, normally at the level of the fourth cervical vertebra6. Hypoglossal nerve is probably the most frequently damaged because of its proximity to the bifurcation7. When the bifurcation is at the level of C3 or above it (close to the mandible branch)6, or in cases of carotid body tumors firmly adhered to the adventitia of the artery or involving them (type III Shamblin tumor)10, surgical exposition is more difficult, and it may require maneuvers such as stylohyoid muscle section, posterior portion of the digastric muscle, styloid process, mobilization of the inferior region of the parotid gland, and mobilization of the mandible to have distal access to the vessel5. In such cases, there is a higher risk of damaging the nerves, because the hypoglossal nerve is close to the bifurcation, that is, it needs more stabilization5, 7.
Direct damage or section of hypoglossal nerve is rare and pathophysiology of the dysfunction is the edema that results from the trauma secondary to retraction and nerve dissection12. The damage may also take place at the level of the bleeding control of the small vessels around the nerve7, 14. Hypoglossal nerve dysfunction may manifest as ipsilateral deviation of the tongue, disarthria, pain, difficulty in mastication and swallowing, and bilateral paralysis of hypoglossal nerve may cause articulation disorders, swallowing disabilities, and even medical emergency leading to obstruction of the airways that requires tracheotomy20. Therefore, when performing bilateral surgery, more attention should be given to the case12.
Although evolution of most lesions is benign with recovery of function between 1 and 6 months14, 24, it may be a significant complaint of the patient or become permanent23. The frequent multiplicity of the lesion of cranial nerves is another important aspect, increasing morbidity2, 24.
Some previously described maneuvers used to help medial and superior mobilization of hypoglossal nerve are division of occipital artery, sternocleidomastoid vein and artery, and section of hypoglossal loop8. Despite the fact that sectioning of the loop of the hypoglossal nerve does not result in sequelae, it may be preserved through posterior dissections to reach the carotid artery15.
There are multiple causes for the high incidence of hypoglossal nerve damage in surgeries of carotid body tumor: adherence and incorporation of the arteries by the tumor5, 9, 17, inappropriate cervical exposition5, 17, difficulty to locate the nerve in relation to the tumor, or the surgeon's lack of interest in preserving it17. The risk is greater in tumors larger than 5cm15, 17, or type III Shamblin tumors10.
To prevent the lesion of the cranial nerve it is important to identify all cranial nerves before tumor dissection3, 17, similarly to appropriate cervical exposition, which can be reached through previously described maneuvers for endarterectomy. In addition, pre-operative embolization, multidisciplinary team, use of modern surgical technique with imaging amplification and bipolar electrocautery, are equally useful to reduce the high incidence3, 9.
Despite the knowledge of the relation of the hypoglossal nerve and the carotid bifurcation and the attention of the surgeon concerning the cranial nerves, there are no other anatomical studies that measured that distance. We did not defined statistical correlation between distance and length of neck, gender, race and age in our sample, but it is important to define some parameters to help locate the hypoglossal nerve. Maybe with a larger sample or by studying other anatomical parameters it would be possible to define that relation. In the present study, we showed that there is great variability of the hypoglossal nerve in relation to the carotid bifurcation and the surgeon should be aware that the hypoglossal nerve may be located close (0.5cm) or some centimeters (4.3cm) above the bifurcation. The hypoglossal nerve was never found under the carotid bifurcation as reported by other studies.
Conclusion
In spite of the great advance in surgical techniques, anatomical knowledge is essential in carotid artery surgery since there are variations of the hypoglossal nerve in relation to the carotid artery. The present anatomical study represents more than clinical impression and despite its simple methodology, it provides objective information about the variability of the hypoglossal nerve and the carotid bifurcation.
References
1. Aldoori, M.I., Baird, R.N. - Local neurological complication during carotid endarterectomy. J Cardiovasc Surg 29:432-6, 1988.
2. Ballota, E.; Giau, G.; Renon, L. et al. - Cranial and cervical nerve injuries after carotid endarterectomy: A prospective study. Surgery 125:85-91, 1999.
3. Borges, L.F.; Heros, R.C.; DeBrun, G. - Carotid body tumors managed with preoperative embolization. J Neurosurg 59:867-70, 1983.
4. Forssel, C.; Kitzing, P.; Bergqvist, D. - Cranial nerve injuries after carotid artery surgery. A prospective study of 663 operations. Eur J Vasc Endovasc Surg 10:445-9, 1995.
5. Hallet, J.W.; Nora, J.D.; Hollier, L.H.; Cherry, K.J.; Pairolero, P.C. - Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: A fifty-year experience with 153 tumors. J Vasc Surg 7:284-91, 1988.
6. Hans, S.S.; Shah, S.; Hans, B. - Carotid endarterectomy for high plaques. Am J Surg 157:431-5, 1989.
7. Hertzer, N.R.; Feldman, B.J.; Beven, E.G.; Tucker, H.M. - A prospective study of the incidence of injury to the cranial nerves by carotid endarterectomy. Surg Gynecol Obstet 151:781-4, 1980.
8. Imparato, A.M.; Bracco, A.; Kim, G.E.; et al. - The hypoglossal nerve in carotid arterial reconstruction. Stroke 3:576-8, 1972.
9. LaMuraglia, G.M.; Fabian, R.L.; Brewster, D.C. et al. - The current surgical management of carotid body paragangliomas. J Vasc Surg 15:1038-45, 1992.
10. Lees, C.D.; Levine, H.L.; Beven, E.G.; Tucker, H.M. - Tumors of the carotid body. Am J Surg 142:362-5, 1981.
11. Leonneti, J.P.; Donzelli, J.J.; Littooy, F.N.; Farrel, B.P. - Perioperative strategies in the management of carotid body tumors. Otolaryngol Head Neck Surg 117: 111-5, 1997.
12. Maniglia, A.J.; Han, P. - Cranial nerve injuries following carotid endarterectomy: an analysis of 336 procedures. Head Neck 13:121-4, 1991.
13. Massey, E.W.; Heyman, A.; Utley, C.; Haynes, C.; Fuchs, J. - Cranial nerve paralysis following carotid endarterectomy. Stroke 15:157-9, 1984.
14. Matsumoto, G.H.; Cossman, D.; Callow, A.D. - Hazards and safeguards during carotid endarterectomy. Am J Surg 133:458-62, 1977.
15. Meyer, F.B.; Sundt, T.M.; Pearson, B.W. - Carotid body tumors: a subject review and suggested surgical approach. J Neurosurg 64:377-85, 1986.
16. Mitchell, R.O.; Richardson, D.; Lambert, G.E. - Characteristics, surgical management, and outcome in 17 carotid body tumors. Am Surgeon 62:1034-7, 1996.
17. Netterville, J.L.; Reilly, K.M.; Robertson, D.; Reiber, M.E.; Armstrong, W.B.; Childs, P. - Carotid body tumors: A review of 30 patients with 46 tumors. Laryngoscope 105:115-26, 1995.
18. Padberg, F.T.; Cady, B.; Persson, A.V. - Carotid Body tumor. Am J Surg 145:526-8, 1981.
19. Ranson, J.H.C.; Imparato, A.M.; Claus, R.H.; Reed, G.E.; Hass, W.K. - Factors in the mortality and morbidity associated with surgical treatment of cerebrovascular insufficiency. Circulation 39 (suppl. I):269-74, 1969.
20. Rutherford, R.B. - Atlas of vascular surgery. Basic techniques and exposure. W.B. Saunders Company, Philadelphia, Pennsylvania, 1993.
21. Schauber, M.D.; Fontenelle, L.J.; Solomon, J.W.; Hanson, T.L. - Cranial/cervical dysfunction after carotid endarterectomy. J Vasc Surg 25:481-7, 1997.
22. Skillman, J.J.; Kent, K.C.; Anninos, E. - Do neck incisions influence nerve deficits carotid endarterectomy? Arch Surg 129:748-52, 1994.
23. Verta, M.J.; Applebaum, E.L.; McClusky, D.A.; Yao, J.S.T.; Bergan, J.J. - Cranial nerve injury during carotid endarterectomy. Ann Surg 185-192-5, 1977.
24. Weiss, K.; Kramar, R.; Firt, P. - Cranial and cervical nerve injuries: Local complications of carotid artery surgery. J Cardiovasc Surg 28:171-17, 1987.