Year: 2002 Vol. 68 Ed. 1 - (6º)
Artigo Original
Pages: 34 to 38
Repeatability of evoked otoacoustic emissions in normally hearing adults
Author(s):
Mariana C. Guedes 1, Simone N. Passos 1,
Maria Valéria S. Goffi Gomez 2,
Ricardo Ferreira Bento 3
Keywords: otoacoustic emissions, transient evoked otoacoustic emissions, distortion product otoacoustic emissions, test-retest reliability.
Abstract:
Introduction: some studies report the importance of the cochlear function monitoring when dealing with patients exposed to ototoxic drugs or agents. A lot of research about test-retest reliability of the otoacoustic emissions was done to validate its use on cochlear monitoring. Nevertheless, our clinical experience shows us that the measured variations differ from those described in the literature. Aim: verify the variation of the transient evoked otoacoustic emissions and the distortion product otoacoustic emissions. Study design: clinical prospective randomized. Material and method: we studied 10 normal hearing young adults, using the Madsen, Celesta 503 program. Three serial evaluations were done, with a minimal interval of three weeks, maintaining variables such as olive size, number of sweeps and stimulus intensity controlled. Results: showed that the transient evoked otoacoustic emissions amplitude varied less than distortion product otoacoustic emissions amplitude and that the amplitude of the initial response was not determinant in the amount of variability.
![]()
1 Clinical Audiologist. Former Participant of Theoretical-Practical Course on Clinical Audiology, Division of Clinical Otorhinolaryngology, HCFMUSP.
2 Speech and Language Therapist, Sector of Speech and Language Therapy, Division of Clinical Otorhinolaryngology, HCFMUSP, Ph.D. in Human Communication Disorder Sciences, UNIFESP/EPM.
3 Associate Professor, Discipline of Otorhinolaryngology, FMUSP.
Study conducted at the Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School of University of São Paulo.
Address correspondence to: A/C Maria Valéria S. Goffi Gomez - Av. Dr. Enéas Carvalho de Aguiar nº 255 Clínica Otorrinolaringológica do HCFMUSP 6º Andar - ICHC
Article submitted on October 10, 2001. Article accepted on November 06, 2001.
INTRODUCTION
Otoacoustic emissions (OAE) are sounds generated inside the normal cochlea, either spontaneously or as a response to an acoustic stimulation (Norton and Stover, 1994). The first cochlear responses were recorded by David Kemp from the Institute for Laryngology and Otology (ILO) in London, in 1978 (Kemp, 1978).
Different studies confirmed the cochlear origin of otoacoustic emissions. Some said that otoacoustic emissions could be measured when neural response was absent (Robinette and Durrant, 1997), others that emissions were absent when the cochlea was affected by ototoxic drugs (Zorowka et al., 1993), or by hypoxia (Lonsbury-Martin, 1987). Thus, although there are still many unanswered questions concerning the cochlear function and otoacoustic emissions, there is a growing interest in otoacoustic emissions because it is a quick, precise and non-invasive method to investigate the cochlear function (White et al., 1993; Norton and Stover, 1994).
There are two basic types of otoacoustic emissions resulting from the inner ear activity: spontaneous emissions, that may be recorded without presenting sound stimuli and evoked emissions, which are recorded secondarily to the presence of a sound. They are divided into transient evoked otoacoustic emissions (TEOAE), distortion product otoacoustic emissions (DPOAE) and stimulus-frequency otoacoustic emissions, still with no clinical application.
TEOAE are evoked by clicks and they are present in practically all healthy ears. TEOAE are clinically used in auditory screenings, diagnosis of retrocochlear pathologies and monitoring of cochlear function (Gattaz and Cerruti, 1994; Bento et al., 1998; Choi et al., 1999).
Distortion product otoacoustic emissions are acoustic energy originated in the cochlea by the non-linear interaction of two pure tones applied simultaneously, thus we can analyze the cochlear activity in specific frequencies. Transient evoked otoacoustic emissions are responses produced by the activity of the outer hair cells inside the cochlea, provoked by a very brief sound (click) and they represent the global cochlear response (Lopes Fo. et al., 1996; Hussain et al., 1998; Hotz et al., 1994).
When all hearing thresholds from 250 Hz to 8000Hz are better than 20dB, TEOAE are present in 99% of the ears. TEOAE responses consist of multiple source components and they interact in a very complex fashion to provide the final result (Harris e Probst, 1997).
Otoacoustic emissions are transmitted from the cochlea through the ossicles and the tympanic membrane (TM) and they are measures in the external auditory canal. Any middle ear (ME) abnormality or obstruction of the external acoustic canal may potentially interrupts the transmission of TEOAE. Therefore, it is recommended that testing of TEOAE be complemented with immitanciometry (Choi et al., 1999). Thresholds greater than 30 dB (HL) do not show transient otoacoustic emissions, because of failure of outer hair cells and lack of enough intensity to stimulate them. Conversely, in hearing losses up to 45 dB HL, it is possible to detect distortion product responses (Miniti et al., 2000).
Since its discovery, OAE study has been widely used in neonatal screening, as a support for the diagnosis of neural conditions, to follow-up cochlear function in treatments with ototoxic drugs or exposure to harmful cochlear agents (Robinette and Glattke, 1997).
Some authors studied repeatability of otoacoustic emissions to validate their use in monitoring of cochlear function (Harris et al., 1991; Vedantam and Musiek, 1991; Franklin et al., 1992; Roede et al., 1993), however, clinical experience has shown us that the variation values of test-retest do not agree with the ones reported in the literature.
The present study intended to study and quantify the individual variations of relative amplitude of transient evoked and distortion product otoacoustic emissions in normal hearing subjects.
MATERIAL AND METHOD
The present study was conducted in the sector of Speech Therapy and Audiology of the Division of Clinical Otorhinolaryngology, Medical School, University of São Paulo. We analyzed the results of transient evoked otoacoustic emissions (TEOAE) and distortion product otoacoustic emissions (DPOAE) in 10 subjects, aged 22 to 38 years, with no hearing disorders or history of otological affections, no exposure to occupational noise and absence of ototoxic drug treatment.
To participate in the study, all subjects had normal hearing thresholds (up to 25 dB HL in all tested frequencies, from 250 to 8000Hz), normal tympanometric curves and presence of stapedial reflexes up to 100 dBHL in all tested frequencies (500Hz to 4000Hz) in both ears.
Investigation of thresholds was performed by descending method, using the audiometer Madsen Midimate 622. The device Interacoustics AZ 26 was used for the impedanciometry.
Otoacoustic emissions were studied in a acoustically treated room, with equipment CELESTA 507, Madsen.
Each subject was submitted to three different tests of TEOAE and DPOAE, within a 4-week interval between each one.
Before the beginning of each test, we performed the check fit. TEOAE were studied with non-linear stimulus at 80dB, with 1,000 samples. The criterion for presence of response was a correlation of at least 0.50 between the responses (reproducibility) and amplitude of global response of at least 3dB ((S/N > 3 dB).
DPOAE were studied with stimulus f1/f2 at 70dB (relation 1.2) and the test was concluded at 100ms or 12.0dB of amplitude per frequency. We analyzed frequency responses to 1000, 2000, 4000, 6000 and 8000Hz. The response to DP1 (2f1-f2) was considered present if the emission amplitude exceeded 6dB of noise level (S/N > 6 dB).
We maintained constant the test conditions subject to control such as size of tip, number of presentations and intensity.
RESULTS
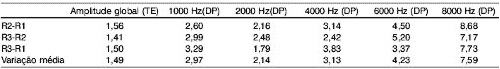
We analyzed the variation of responses for each performed test, within 4-week intervals. The mean variation, in dB, of each test is represented in Table 1, both for transient and distortion product otoacoustic emissions in each tested frequency.
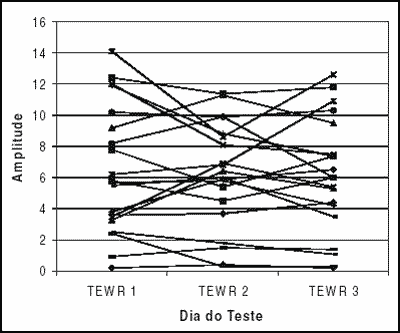
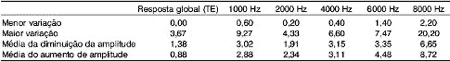
In Graph 1, we can observe the amplitude variation of responses to transient evoked otoacoustic emissions in tested subjects in each test performed.Graph 1. Responses from 20 tested ears in each of the three assessments.Table 1. Variation of amplitude of response of transient evoked and distortion product otoacoustic emissions (in dB) in different tested frequencies in the three assessments.Table 2. Minimum and maximum values of variation, mean improvement or worsening of responses of transient and distortion product otoacoustic emissions (in dB) in different tested frequencies in all assessments.
Upon analyzing the results of transient otoacoustic emissions, the mean variation found was 1.49 dB (ranging from 0 to 3.67, standard deviation of 1.1 and median of 1.5). In the analysis of distortion product otoacoustic emissions, we found the mean variation of 2.97dB in 1000Hz, 2.14dB in 2000Hz, 3.13dB in 4000Hz, 4.23dB in 6000Hz and 7.59dB in 8000Hz, with standard deviations of 2.06; 1.19; 1.74; 1.94 and 4.48 respectively. Data are presented in Table 2.
DISCUSSION
Probably, the most remarkable benefit of OAE is its capacity to study in an objective and non-invasive fashion the first stages of sound processing at the level of the biomechanical activity of the OHC. The susceptibility of this cellular receptor to adverse effects of viral and bacterial diseases, genetic alterations, external agents, such as loud noise, ototoxic and chemical drugs, is very well known, since all these factors damage hearing. It was the extraordinary selective and sensitive property of "lesion location" of produced responses that encouraged the researchers to turn procedures into clinical application methods to approach the initial stages of the hearing process (Lonsbury-Martin et al., 1995).
To monitor hearing, it is necessary to determine reliable parameters about variation of response in normal subjects so that they may be applied to cases of patients treated with ototoxic drugs, those that make use of harmful chemicals or patients exposed to high levels of noise.
As to use of ototoxic drugs, Zorowka et al. (1993) observed reduced amplitude of TEOAE in 8 children that used cisplatin when compared to the control group and Hotz et al. (1994) found amplitude of about 3.2dB lower in subjects that used aminoglycoside (amicacyn sulfate) in TEOAE.
Franklin et al. (1992) found a variation of approximately 2dB in amplitude of TEOAE in normal subjects (n = 12). In the present study, TEOAE presented a variation of 3.69dB (considering mean + 2SD).
Harris et al. (1991) found a variability of 2dB in a study with 10 normal subjects using the ILO 88 analyzer. The difference could be explained by the use of different programs for generation and recording of OAE. Similarly to those authors, in the present study, we observed variability of responses not related to amplitude, that is, small responses continued to be small and those responses with higher amplitude, remained so.
Vedantam and Musiek (1991) found high correlation of test-retest in OAE (p<0.0001), but the interval between the tests was of only one hour and 30 minutes, which enabled more control over the variation of general physical status of the subjects and environment conditions.
It is known that alterations in middle ear pressure may cause reduction of amplitude of response; lack of sensitivity of the tip in the external auditory canal would interfere in recording of OAE. Therefore, we tried to control parameters are much as possible during the performance of the test and the recording of responses, such as noise, middle ear conditions and placement of the tip into the external auditory canal, however, we believe that there are other factors that could interfere in the recording of responses and their amplitudes, which can not be strictly controlled by the researcher in the clinical situation.
The variation of clinical amplitude was also analyzed in DPOAE and more variation was found in amplitude of high frequencies (6000 and 8000Hz) than in frequencies of 1000 and 2000Hz, exactly the frequencies that are initially affected by ototoxic agents, chemicals and noise, which are those that show early the involvement of outer hair cells. According to the studies by Roede et al. (1993), a variation of 6 to 9dB in amplitude of DPOAE could indicate a significant change of cochlear dynamics, provided that the middle ear conditions are maintained.
Franklin et al. (1992) found an average variation of 2dB in amplitude of middle frequencies of DPOAE and a variation greater than 2 dB in extreme frequencies (1000 and 8000Hz). Littman et al. (1998) observed absence of DPOAE responses after subjects had taken cumulative doses of cisplatin, but before worsening of pure tone thresholds.
Comparing the responses of TEOAE and DPOAE, we noticed a reduced variability of amplitude concerning transient otoacoustic emissions than amplitude of distortion product otoacoustic emissions.
Responses varied for the better (increase in amplitude) and for the worse (reduction in amplitude), but no studied subjects presented amplitude below 3dB in TEOAE or 6dB in DPOAE in any of the tests.
Based on our findings, we believe it is crucial to analyze other parameters and variables, such as absolute amplitude and noise level of each test. The present study shall be continued with the analysis of such parameters and the statistical study of a larger sample.
CONCLUSION
Based on the results of the present study about variation of relative amplitude of response to otoacoustic emissions in normal subjects, we concluded that, in the studied population:
· TEOAE varied less than DPOAE in normal subjects;
· Mean variation of TEOAE was of 3.69dB (2SD);
· The smallest variation of DPOAE affected the frequency of 2000Hz and it was 2.14dB;
· DPOAE variation was higher in frequencies of 6000 and 8000Hz.
REFERENCES
1. BENTO, R.F.; MINITI, A.; MARONE, S. - Tratado de Otologia. São Paulo. Edusp. 1998 p. 107-16.
2. CHOI, S.S.; PAFITIS, I.A.; ZALZAL, G.H.; HERER, G.R.; PATERL, K.M. - Clinical Applications of Transiently evoked otoacoustic emissions in the pediatric population. Ann Otol Rhinol Laryngol 108:132-8. 1999.
3. ENGDAHL, B.; ARNESEN, R.; MAIR, I. - Reproducibility and short-term variability of transient evoked otoacoustic emissions. Scandinavian Audiology 23:99-104. 1994.
4. FRANKLIN, D.J.; McCOY, M.J.; MARTIN, G.K.; LONSBURY-MARTIN, B.L. - Test-retest reliability of distortion-product and transiently evoked otoacoustic emissions. Ear and Hearing 13 (6):417-29. 1992.
5. Gattaz, G.; Cerruti, V.Q. - O Uso do Registro de Emissões Otoacústicas Evocadas para Triagem Auditiva em Neonatos de Risco para Deficiência Auditiva. Revista Paulista de Pediatria 12 (3):291-4. setembro 1994.
6. HARRIS, F.P.; PROBST R. - Otoacoustic Emissions and Audiometric Outcomes. IN: ROBINETTE, M.S. e GLATTKE, T.J. Otoacoustic Emissions. Clinical Applications. Thieme. New York. 1997. Cap. 8.
7. HARRIS, F.P.; PROBST, R.; WENGER, R. - Repeatability of transiently evoked otoacoustic emissions in normally hearing humans. Audiology 30:135-41. 1991.
8. Hotz, M.A. Harris, F.P. e Probst, R. - Otoacoustic Emissions: An Approach for Monitoring Aminoglycoside-Induced Ototoxicity. Laryngoscope 104:1130-4. 1994.
9. Hussain, D.M.; Gorga, M.P.; Neely, S.T.; Keefe, D.H.; Peters, J. - Transient Evoked Otoacoustic Emissions in Patients with Normal Hearing and in Patients with Hearing Loss. Ear Hear: 434-49. 1998.
10. KEMP, D.T. - Development in cochlear mechanics and techniques for noninvasive evaluation. Advanced Audiology 5:27-45. 1988.
11. KEMP, D.T. - Stimulated acoustic emissions from within the human auditory system. Journal of the Acoustic Society of America 64:1386-91. 1978.
12. LITTMAN, T.A.; MAGRUDER, A.; STROTHER, D.R. - Monitoring and predicting ototoxic damage using distortion-product otoacoustic emissions: Pediatric case study. J. Am Acad Audiol 9:257-62. 1998.
13. LONSBURY-MARTIN, B..L. - Acoustic distortion products in rabbits. I. Basic features and physiological vulnerability. Hear Res 28:173-6. 1987.
14. LONSBURY-MARTIN, B..L.; MARTIN, G.K.; MC COY, M.J.; WHITEHEAD, M.L. - New approaches to the evaluation of the auditory system and a current analysis of otoacoustic emissions. Otoalryngol Head Neck Surg 112:50-63. 1995.
15. Lopes Filho, O.; Carlos, R.; Thomé, D.; Eckley, C. - Emissões Otoacústicas Transitórias e Produtos de Distorção na Avaliação da Audição em Recém-Nascidos com Poucas Horas de Vida, Revista Brasileira de Otorrinolaringologia 62 (3):220-8. 1996
16. MINITI, A.; BENTO, R.F.; BUTUGAN, O. - Otorrinolaringologia. Clínica e Cirúrgica. 2ª edição. São Paulo. Atheneu. 2000. Cap.10.
17. NORTON, S.J. e STOVER, L.J. - Otoacoustic Emissions: An emerging tool. IN: KATZ, J. Handbook of Clinical Audiology. 4Th. edition. William and Wilkins. Baltimore. 1994.
18. ROBINETTE, M.S. e DURRAN, J.D. - Contributions of evoked otoacoustic emissions in differential diagnosis of retrocochlear disorders. IN: ROBINETTE, M.S. e GLATTKE, T.J. Otoacoustic Emissions. Clinical Applications. Thieme. New York. 1997.
19. ROBINETTE, M.S. e GLATTKE, T.J. - Otoacoustic Emissions. Clinical Applications. Thieme. New York. 1997.
20. ROEDE, J.; HARRIS, F. P.; PROBST, R; XU, L. - Repeatability of distortion product otoacoustic emissions in normally hearing humans. Audiology 32:273-81 1993.
21. VEDANTAM, R.; MUSIEK, F.E. - Click evoked otoacoustic emissions in adult subjects: standard indices and test-retest reliability. Am J Otology 12 (6):485-442. 1991.
22. White, K.R.; Vohr, B.R.; Behrens, T.R. - Universal Newborn Hearing Screening Using transient evoked otoacoustic emissions: Results of the Rhode Island Hearing Assessment Project. Seminars in Hearing 14 (1):18-29. 1993.
23. ZOROWKA, P.G.; SCHMITT, H.J.; GUTJAHR, P. - Evoked otoacoustic emissions and pure tone threshold audiometry in patients receiving cisplatinum therapy. Int J Pediatric Otorhinolaryngol 25:73-80. 1993.