Year: 2004 Vol. 70 Ed. 5 - (12º)
Artigo Original
Pages: 658 to 663
Effects of mitomycin C in stromal culture of eosinophilic nasal polyps: induction of apoptosis in eosinophils.
Author(s):
Paulo Fernando Tormin Borges Crosara1,
Evaldo Nascimento2,
Sebastião C. Sobrinho3,
Anilton C. Vasconcelos4,
Roberto E. S. Guimarães3,
Helena M.G. Becker3,
Enrico A. Calosimo5,
Flávio B. Nunes6
Keywords: nasal polyps, eosinophils, mitomycin C, apoptosis.
Abstract:
The eosinophilic nasal polyposis occurs as a manifestation of several diseases. It affects multiple nasal sinuses and presents with high relapsing rates after current treatment. The eosinophils play an essencial role in the pathogenesis through their low apoptosis rates and the long periods of remaining in the tissues in an active form. Objective: During the present study the effect of C-mitomycin was observed in the apoptosis induction of stromal eosinophils in eosinophilic nasal polyps. Materials and Methods: During this auto-matched study, 9 samples of eosinophilic nasal polyps were cultivated in RPMI 1640 medium and then evaluated at the beginning, and after 12 and 24 hours of incubation. The study group was submitted to C-mitomycin in a 400 µg/ml concentration during 5 minutes. Both control and study group cultures were evaluated histopathologically and their apoptotic rates were determined. They were stained using hematoxilin-eosin and observed through microscopic enhancement of 1000 times. Results: The analysis of 674 digitalized fields showed that those cultures treated with C-mitomycin achieved significantly higher apoptotic rate after 12 hours of incubation when compared to control group rate (p<0,001). Conclusion: This study supports that C-mitomycin is efficacious in the induction of the apoptosis of stromal eosinophils in eosinophilic nasal polyps.
![]()
Introduction
Nasal polyposis (NP) is the presence of multiple nasal polyps, usually bilaterally, in the nasal mucosa, due to inflammation. Histologically, nasal polyps may be eosinophilic or non-eosinophilic. Eosinophilic NP represents 80 to 90% of all NPs. It is associated with nasal and/or bronchial hyperactivity and presents good response to corticosteroids. It affects patients with upper and lower airway diseases caused by different etiologic factors and in different stages of the disease. Eosinophilic NP may occur in Non-Allergic Eosinophilic Rhinitis (NARES), Aspirin intolerance, Atopy, Allergic Fungal Sinusitis and Churg Strauss Syndrome.1,2,3
Eosinophil infiltration in polyps is the most remarkable feature of eosinophilic NP, and this is important for our understanding of the disease's pathogenesis. Tissue eosinophilia may be explained by increased eosinophil migration to the inflamed area and/or increased survival of eosinophils in tissues, as a result of decreased apoptosis.4
Apoptosis, or programmed cell death, refers to a non-pathologic process of cell death. It is characterized by a chain of events that are different from necrosis, preventing the release of cell substances and the activation of inflammatory mediators.5
Apoptosis is an active process, dependent on RNA and protein synthesis. Simon et al. (1997)6 demonstrated that granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin 5 (IL-5), produced by eosinophils and lymphocytes 7,8, increase the survival of eosinophils through apoptosis suppression.
Bjornson, Harvey and Rose (1985)9 demonstrated that hydrocortisone acts directly on eosinophils to suppress colonies. Liles, Dale and Klebanoff (1995)10 and Meagher et al. (1996)5 demonstrated an opposite effect of corticosteroids on eosinophils and neutrophils. Neutrophils would increase in numbers due to the reduced apoptotic rate.11,12
Fas receptor (CD 95/APO1), member of TNF/NGF superfamily (Neural Growth Factor) seems to stimulate apoptosis in eosinophils. Its activation in eosinophils from normal individuals promptly induces apoptosis. On the other hand, eosinophils from eosinophilic individuals do not suffer apoptosis, even when the active Fas receptor is normally expressed in these cells.13 Therefore, there are possibly other systems involved in apoptosis regulation.14,15
Whyte, Meagher and Haslett (1991)16 proved that blockage in protein synthesis through cycloheximidine and actinomycin-D induces apoptosis in eosinophils and neutrophils, unlike corticosteroids. They concluded that apoptosis is constantly suppressed by the synthesis of certain proteins and induced when these proteins are absent.
Mitomycin C (MMC) is an antibiotic and antineoplastic agent extracted from Streptomyces caespitosus. Its mechanism of action is selective suppression of DNA synthesis, recombination, change of sister chromatins and DNA fixation in bacteria.17
There are few reports of the induction of apoptosis in mammal cells using MMC. Schwartz et al. (1995)18 reported the induction of apoptosis in gastric tumors treated with MMC. A single application of MMC, 0.4mg/ml, for five minutes is able to suppress proliferation in fibroblast culture for 35 days.19 Kim et al. (1999)20 noted that, in vitro, MMC (0.4 mg/ml for 5 minutes) induced apoptosis in Tenon's capsule of human fibroblasts, primarily cultured.
This drug has been largely used in laryngology and otology, with good results and safety profile. 21,22,23,24
The purpose of the present study was to evaluate the effects of Mitomycin C on the apoptotic rate of stromal eosinophils of eosinophilic nasal polyps.
MATERIAL AND METHOD
The study population included patients, aged 16 to 79 years, extensively affected with NP, referred through the Universal Healthcare System (Sistema Único de Saúde SUS) for surgical treatment at Hospital São Geraldo, an annex of Hospital das Clínicas, Federal University of Minas Gerais HC-UFMG.
Inclusion criteria: patients affected by eosinophilic nasal polyposis with percentage equal to or over 40%, regardless of the etiology.
Exclusion criteria: unilateral nasal polyps, purulent discharge and use of drugs such as corticosteroids or antihistamines for 2 months before the initial examination.
Nine patients with eosinophilic NP were selected; they had eosinophil percentages equal to or over 40%25, from January to June 2003.
The selected patients were subject to thorough otorhinolaryngologic examination, focusing the nasal area. Nasal cavities were examined through anterior rhinoscopy and laryngoscopy.
The self-paired comparative experimental study was conducted in 9 samples of eosinophilic nasal polyps of approximately 3 mm. The study group included cultures of polyp fragments from each patient treated with MMC and examined at 0, 12 and 24 hours. The control group included cultures of polyp fragments not treated with MMC, examined at the same time periods.
Nasal polyps' biopsy collected for culture was processed at the Otorhinolaryngologic outpatient facility of HC-UFMG. The collected fragments were immediately put in culture medium at room temperature. Two FALCON® tubes were used for collection. The sample was put into the first tube for 1-2 minutes, slightly stirred and transferred to the second tube. The purpose of this procedure was to reduce culture contamination.
At the Immunology Laboratory of Hospital São Geraldo, the fragments were cut into 6 parts and seeded in a 6-orifice plate. Three orifices were for the test group and three for the control group. The test group received 400 µg/ml19 of MMC for five minutes. After drug application, the cultures were washed in RPMI medium. For the control group, not treated with MMC, we used only the RPMI medium. Fragments from the first two orifices, test and control, were immediately prepared for histopathologic examination. The other two pairs of samples, each one with test and control samples, were incubated in culture medium for 12 and 24 hour, respectively, in oven, at 370 C and 5% of CO2. At the end of each time (12 and 24 hours), fragments were sent for histopathologic examination.26
The culture medium used was RPMI 1640, traded by Gibco UK26, composed of 5% AB human serum, 2 M of mercaptoethanol, 1 mM of L-glutamine, 2mM of sodium piruvate, 10 gr/ml of streptomycin, 100U/ml of penicillin and 5mg/ml of fungizone.
After removal of the supernatant, the fragments of each sample were transferred to flasks filled with 10% formalin, embedded in paraffin and sent for histopathologic examination. The glass slides with samples were stained in hematoxylin-eosin (HE) and examined under light microscope. Images of fields with eosinophilic infiltrate were digitalized, stored in compact disc and subsequently analyzed by morphometric study. This part of the study was conducted in the Laboratory for Apoptosis and Morphometry (Department of General Pathology, ICB - UFMG).
Cell count analysis, i.e., tissue eosinophilia, was done under light microscope, through scanning of 5-10 fields, using magnification of 400X. In order to obtain the percentage of eosinophils, we counted the highest number of inflammatory cells .25
To analyze the apoptotic rate (AR) in each glass slide, we counted eosinophils under magnification of 1,000X, and divided all apoptotic eosinophils by the counted eosinophils. 4 The apoptotic eosinophil is characterized by loss of cell adhesion, cytoplasmatic and nuclear condensation and nuclear coalescence or extrusion.4,27
The method used for statistical analysis was paired t-test for comparison of groups in time, according to Snedecor et Cochran (1977)28. We considered a level of significance of 5% and used Minitab 13, for analysis of the statistical data.
This study was previously approved by UFMG's Ethics Committee, Resolution No. 109/02.
RESULTS
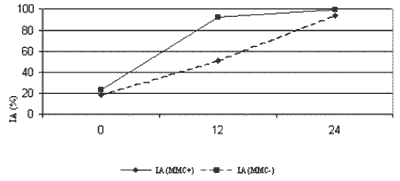
Nine tests were sent for slide preparation and calculation of apoptotic rate (Figure 1).
The analysis resulted in 741 digitalized fields, and 67 fields were examined under magnification of 400X for cell count. Cell count average (eosinophil percentage) was 55.7% (48.2%-67.5%). To determine AR, we examined 674 fields under 1,000X magnification. The results of AR analysis of the 9 tests are shown in figure 1. At time 0, there was no significant difference between both groups, treated with MMC or untreated (p= 0.189). At time 12, the treated cultures had apoptotic rate significantly higher then the untreated samples (p<0.001). At time 24, there was no significant difference between the groups (p= 0.301).
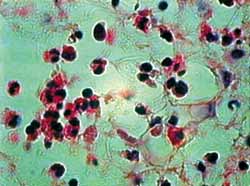
In single analysis of the untreated group, we can note significant difference between times 0 and 12 (p<0.001), times 0 and 24 (p<0.001) and times 12 and 24 (p<0.001). This same pattern is maintained in the group treated with MMC, where there was significant difference between times 0 and 12 (p<0.001), times 0 and 24 (p<0.001) and times 12 and 24 (p< 0.001). As shown in figures 2 to 5, the method used for histopathologic examination is highly promising for detection of apoptotic eosinophils.
Figura 1. IA nas culturas tratadas e não tratadas com MMC. IA (MMC-) refere-se às culturas sem MMC. IA (MMC+) refere-se às culturas com MMC.
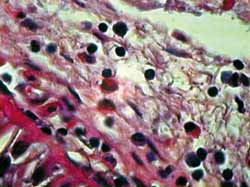
Figura 2. Corte hispatológico sem aplicação de MMC, no tempo zero hora. Eosinófilos se apresentam viáveis em sua maioria. Coloração pela HE. Aumento de 1.000X.
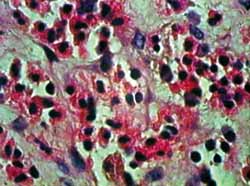
Figura 3. Corte hispatológico com aplicação de MMC, no tempo zero hora. Como no corte anterior (Figura 2), os eosinófilos mantem-se viáveis em sua maioria. Coloração pela HE. Aumento de 1.000X.
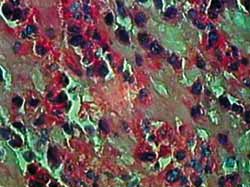
Figura 4. Corte hispatológico sem aplicação de MMC, no tempo de 12 horas. Os eosinófilos mantem-se viáveis em sua maioria, mas alguns já apresentam sinais de apoptose. Coloração pela HE. Aumento de 1.000X.
Figura 5. Corte hispatológico com aplicação de MMC, no tempo de 12 horas. Os eosinófilos já mostram evidentes sinais de apoptose. Nota-se, também, desorganização do tecido conjuntivo. Coloração pela HE. Aumento de 1.000X.
DISCUSSION
Staining with HE provides clear visualization of eosinophils and histopathologic changes of apoptosis. As shown in figures 2 to 5, we can clearly note loss of cell adhesion, nuclear and cytoplasmatic shrinkage and nuclear coalescence and extrusion. We could detect many apoptotic bodies (cytoplasmatic and/or nuclear extrusion) in the 12 hour-sections treated with MMC and the 24 hour-sections either treated with MMC or untreated. This result shows that the method used was clearly beneficial for histopathologic examination of this type of tissue damage.27
Apoptosis or programmed cell death is a process to regulate inflammation. It is believed that in eosinophilic NP, apoptosis suppression would result in late removal of eosinophils from tissues; consequently, the eosinophils would remain active, increasing eosinophilia and tissue damage.6,8
Some studies show that high levels of IL-5 and GM-CSF suppress apoptosis. Davidsson, Anderson and Hellquist (2000)13 showed that when eosinophils are active in eosinophilic NP, the first sign of the apoptotic cascade is the active Fas antigen. Consequently, apoptosis should be constantly suppressed by protein synthesis. Dibbert et al. (1998)12 showed that IL-5 and GM-CSF stimulate anti-apoptotic gene Bcl-xL. On the other hand, the role of pro-apoptotic gene BAX does not change in the presence of these cytokines.
Cox (1995)11 and Meagher et al. (1996)5 concluded that while using corticosteroids, apoptosis suppression would occur due to the synthesis of specific proteins, not through GM-CSF direct effect. A proof of the need of protein synthesis to suppress apoptosis was provided by Whyte, Meagher and Haslett (1991)16, who induced apoptosis in eosinophils and neutrophils through the use of cycloheximidine and actinomycin-d.
MMC is an alkylating antiblastic agent able to suppress DNA synthesis17 and it induces apoptosis in gastric tumor cells.18 Kim et al. (1999)20 noted that in vitro MMC (0.4 mg/ml for five minutes) induced apoptosis in Tenon's capsule of human fibroblasts.
MMC has been largely used to prevent scars in multiple fields of medicine.22 One single application of MMC, 0.4 mg/ml for five minutes suppresses proliferation in a fibroblast culture for 35 days.19 Jang, Song and Pack (2003)24 studied the effects of MMC, 400µg/ml, on fibroblast culture of tympanic membrane and concluded that there is statistically significant difference in suppression of proliferation, compared to control group. These studies support the use of MMC, 0.4 mg/ml, in our study.
AR analysis did not show significant differences at time 0 for the study groups, untreated or treated with MMC (p= 0.189). At time 12, there was statistically significant difference between groups (p< 0.001). This result supports MMC's effects as alkylating agent, suppressing protein synthesis and releasing the apoptotic cascade, resulting in morphologic changes and cell death. These changes were clearly detected through histopathologic examination.
At time 24, there was no statistically significant differences between groups, p= 0.301. This results from high AR in both groups. In the untreated group, AR increased progressively and slowly, whereas in the group treated with MMC, there was fast progression in AR during the first 12 hours.
We believe that MMC, as an alkylating agent, would block protein synthesis in the involved cells, thus suppressing the anti-apoptotic mechanisms and releasing pro-apoptotic mechanisms induced in eosinophil activation13, but keeping in constant blockage through the effects of cytokines, such as GM-CSF and IL-5.16 Therefore, after MMC application, the levels of GM-CSF in the tissues would be low, suppressing the activation of more eosinophils and releasing the apoptotic cascade in eosinophils affected by the drug.5,7,12
We noticed that in 24 hours, there was high percentage of apoptotic eosinophils in cultures of the untreated group, reaching the same levels as in cultures from the group treated with MMC at time 24. This is possibly due to the absence of stimulating factors.
CONCLUSIONS
The data in this study showed that mitomycin C caused increase in apoptotic rate in stromal cultures of eosinophilic NP.
REFERENCES
1. Settipane GA, Nasal polyps and systemic diseases. In: SCHATZ, M.; ZEIGER, R.S.; SETTIPANE, G.A. Nasal manifestations of systemic diseases. Providence: Oceanside Publ., p.43-51, 1991a.
2. Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, Roberts GD. The diagnosis and incidence of allergy fungal sinusitis. Mayo Clin. Proc., v. 74, n. 9, p. 877-84, Sept. 1999.
3. Becker HMG, Guimarães RES, Nascimento E., Becker CG, Gonçalves DU, CROSARA PFTB. Perfil das Citocinas e Tipificação de HLA em Pacientes com Polipose Nasossinusal Eosinofílica Tolerantes e Intolerantes a Aspirina. Rev Bras Otorrinolaringol. São Paulo: , v.69, n.3, p.296 - 302, 2003.
4. Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ. Eosinophils apoptosis and the resolution of airway inflammation in asthma. Am. J. Respir. Crit. Care Med., v. 154, p. 237-43, 1996.
5. Meagher LC, Cousin JM, Seckl JR, Haslet C. Opposing Effects of Glucocorticoids on the Rate of Apoptosis in Neutrophil Granulocytes. Journal of Imunnology,1996
6. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser C. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. The Journal of Immunology, v. 158, p. 3.902-8, 1997.
7. Simon HU. Regulation of eosinophil and neutrophil apoptosis - Similarities and differences. Immunol. Rev., v. 179, p. 156-62, 2001.
8. Kowalski ML, Grzgorczyc J, Pawliczak R, Kornatowzki T, Wagowzka-Danilewicz M, Danilewicz M. Deceased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivity. Allergy, v. 57, n. 6, p. 493-500, 2002.
9. Bjornson BN, Harvey JM, Rose L. Differential effects of hydrocortisone on eosinophil and neutrophil proliferation. J. Clin. Invest., v. 76, p. 924-9, 1985.
10. Liles C, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood, v. 86, n. 8, p. 3.181-8, 1995.
11. Cox, G. Glucocorticoids treatment inhibits apoptosis in human neutrophils, separation of survival and activation outcomes. J. Immunol., v. 154, p. 4.719, 1995.
12. Dibbert B, Daigle I, Braun D, Schranz C, Weber M, Blaser C, Zangemeister-Wittke U, Akbar A, Simon HU. Role of Bcl-xL in delayed eosinophil apoptosis mediated by granulocyte-macrophage colony-stimulation factor and interleukin-5. Blood, v. 92, n. 3, p. 778-83, 1998.
13. Davidsson A, Anderson T, Hellquist HB. Apoptosis and phagocytosis of tissue-dwelling eosinophils in nasal polyps. Laryngoscope, v. 110, n. 1, p. 111-6, Jan. 2000.
14. Hebestreit H, Yousefi S, Balatti M, Weber M, Crameri R, Simon D, Hartung K, Schapowal A, Blaser K, Simon HU. Expression and function of FAS on human blood and tissue eosinophils. Eur. J. Immunol., v. 26, p. 1.775-80, 1996.
15. Hebestreit H, Dibert B, Balatti I, Braun D, Schapowal A, Blaser K, Simon HU. Disruption of fas receptor signaling by nitric oxide in eosinophils. J. Exp. Med.,v. 187, n. 3, p. 415-25, Feb 1998.
16. Whyte MKB, Meagher L, Haslett C. Cycloheximide and actinomycin-d programmed cell death (apoptosis) in neuthophils. Clin. Sci., v. 80, p. 5, 1991.
17. Carrano AV, Thompson LH, Stretka DG, Minkler JL, Frong S. DNA crosslinking sister chromatid exchange and specific locus mutation. Mutat. Res., v.. 63, p. 175-88, 1979.
18. Schwartz GK, Haimovtz-Friedman A, Dhupar SK, Ehleiter D, Maslak P, Lau L, Laganzo F.Jr, Kelsen DP, Fucks Z, Alpino AP. Potentiation of apoptosis by treatment with protein kinase-c specific inhibitor safingol in mitomycin c treated gastric cancer cells. J. Nat. Cancer Inst., v. 87, p. 1.394-99, 1995.
19. Khaw PT, Doyle JW, Sherwood MB, Grierson H, Shultz G, Megorray S. Prolonged localized tissue effects from 5 minutes exposures to fluoracil and mitomycin C. Arch Ophtalmol, v. 72, p. 155-61, 1994.
20. Kim JW, Kim SK, Song IH, Kim, IT. Mitomycin C-induced apoptosis in cultured human Tenon's capsule fibroblasts. Korean J Ophthalmol, v. 13, n. 1, p. 7-15, Jun. 1999.
21. Correa AJ, Reinish L, Sanders DL, Huang S, Deriso W, Duncavage JA. Inhibition of subglottic stenosis with mitomycin c in the canine model. Ann Otol Rhinol Laryngol, v. 108, n. 1, p.1.053-60, Nov. 1999.
22. Rahbar R, Valdez TA, Shapshay SM. Preliminary results of intraoperative mitomycin-C in the treatment and prevention of glottic and subglottic stenosis. J Voice, v. 14, n. 2, p. 282-6, June2000.
23. Becker CG, Silva AL, Guimarães RES, Becker HM, Barra IM, Oliveira WD. Tratamento cirúrgico da otite media com efusão: tubo de ventilação versus aplicação tópica de mitomicina C. Rev. Bras. Otorrinolaringol. V.69, n.4, 513-9, jul/ago. 2003.
24. Jang CH, Song CH, Pak SC. Effect of exposure to mitomycin c on cultured tympanic membrane fibroblasts. Int. J. Pediatr. Otorhinolaryngol., v. 67, n. 2, p.173-6, Feb. 2003.
25. Ingels K, Durez JP, Cuvelier C, Cauwenberge PV. Nasal biopsy is superior to nasal smear for finding eosinophils in nonallergic rhinits. Allergy, v. 52, n. 3, p. 338-41, Mar. 1997.
26. Teram LM, Park HS, Djukanovic R, Roberts K, Holgate S. Cultured nasal polyps from nonatopic and atopic patients release RANTES spontaneously and after stimulation with phytohemagglutinin. J Allergy Clin Immunol, v. 100, n. 4, p. 499-504, Oct. 1997.
27. Walsh GM, Dewson G, Wardlaw AJ, Lebi-Shaffer F, Moqbel R. A comparative study of different methods for the assessment of apoptosis and necrosis in human eosinophils. Journal of Immunological Methods, v. 217, p. 153-63, 1998.
28. Snedecor GW and Cochran WG. Statistical Methods, AMES: Iowa State University Press, 1977.
Acknowledgements:
This study was possible due to the cooperation of several people and departments, enabling the creating of a new field of study in our Department.
To Prof. José Renan da Cunha Melo, for his support. He offered me free access to his laboratory in the beginning of my research study.
To Edilene Matias do Amaral, for her great effort and dedication in the laboratorial study. I also thank André Macedo Vale and Eliane Perlatto Moura.
To CAPES, for its support and research incentive.
1) Ph.D., Department of Ophthalmology, Otorhinolaryngology and Speech and Hearing Therapy, Federal University of Minas Gerais.
2) Joint Professor, Department of Parasitology, Federal University of Minas Gerais.
3) Joint Professor, Department of Ophthalmology, Otorhinolaryngology and Speech and Hearing Therapy, Federal University of Minas Gerais.
4) Joint Professor, Department of General Pathology, Federal University of Minas Gerais.
5) Joint Professor, Department of Statistics, Federal University of Minas Gerais.
6) Ph.D. studies under course, Department of General Surgery, Federal University of Minas Gerais.
Study conducted at the Department of Ophthalmology, Otorhinolaryngology and Speech and Hearing Therapy, Federal University of Minas Gerais. Laboratory of Immunology Professor Evaldo Nescient.
Address correspondence to: Dr. Paulo Fernando Tormin Borges Crosara - Rua Bernardo Guimarães, 2053, apto1602, Lourdes - Belo Horizonte/MG -Brasil. Cep: 30.140-082. Tel/Fax: (55 31) 3281-4604 - E-mail: pcrosara@hotmail.com.