Year: 2004 Vol. 70 Ed. 4 - (1º)
Artigo Original
Pages: 439 to 448
Histopathological and immunohistochemical differentiation of epithelial alterations in vocal nodule comparing to polyps and to laryngeal edema
Author(s):
Beatrice M. J. Neves1,
João G. Neto2,
Paulo Pontes3
Keywords: Key words: vocal cord, basement membrane, immunohistochemistry.
Abstract:
Purpose: To evaluate by histological and immunohistochemical methods the epithelial lesions in vocal nodule and correlate with polyp, laryngeal edema and vocal folds without macroscopic lesions. Method: In a retrospective analysis of medical records, twenty-six patients, who underwent microsurgical excision of laryngeal inflammatory lesions (nodules, polyps and laryngeal edema), were identified. Vocal folds without macroscopic lesions were obtained from autopsy. To evaluate epithelial lesions, specimens were stained with H&E, PAS, and with antibodies against laminin and collagen type IV. These specimens were analyzed by light microscopy. Results: Lesions diagnosed as nodules showed more collagen type IV and laminin deposition when compared to polyps (p=0,034 and p=0,036, respectively) and to vocal folds without macroscopic lesions (p=0,019 and p=0,021, respectively). Nodules showed basement membranes thicker than polyps on PAS stain (p=0,102). Laryngeal edema could not be differentiated from nodules, polyps or vocal folds without macroscopic lesions by PAS, laminin or collagen type IV stain (p>0,10). Conclusion: By histological and immunohistochemical techniques to evaluate epithelium of laryngeal inflammatory lesions we conclude that vocal fold nodule differentiate from polyps on the three techniques used to stain basement membrane (PAS, laminin, collagen IV) and from vocal folds without macroscopic lesions on two techniques used (laminin, collagen IV). Laryngeal edema cannot be differentiated from the others laryngeal lesions, neither from vocal folds without macroscopic lesions, when the techniques before described are used.
![]()
INTRODUCTION
Vocal fold is a vibration structure responsible for sound production during phonation. It is fundamentally comprised by muscle and mucosa. Mucosa is formed by lamina propria and stratified squamous epithelium on its free margin. An important structure of the epithelium is the basement membrane, which is in direct contact with lamina propria. There are small blood vessels on the vocal fold mucosa, which run parallel to the free margin. There are also glands in the mucosa.1,2
Lamina propria has three layers: superficial, intermediate and deep. The superficial layer of lamina propria is also known as Reinke's space. It is a loose and flexible layer that vibrates intensely during phonation. Intermediate and deep layers comprise the vocal ligament. Lamina propria is primarily comprised by extracellular matrix, fibroblasts and blood vessels. The extracellular matrix is synthesized by fibroblasts and it is comprised by collagen III, elastin, hyaluronic acid and fibronectin 1, 3, 4. Hirano introduced the concept of "cover-body": according to it, the five histological layers of the vocal folds are grouped into three layers based on the mechanical properties of the vocal folds. Epithelium and superficial layer of lamina propria comprises the cover of the vocal fold. Intermediate and deep layers of lamina propria form a transition, the vocal muscle forms the body. The author considers the epithelium as a thin vibration capsule capable of containing the vocal fold during phonation 5.
The basement membrane is a thin layer of specialized extracellular matrix, synthesized by epithelial cells and fibroblasts of lamina propria. It is part of the epithelium and represents the transition to a superficial layer of lamina propria. Together with the fibrous framework of lamina propria, it forms the support for the vocal fold mucosa. It is an important structure for mucosa vibration, since similarly to lamina propria, it promotes elasticity and at the same time, resistance to the vocal folds 4,6,7. Normal basement membrane cannot be observed in details with hematoxylin-eosin staining, routinely used with optical microscopy. Electron microscopy is the best way to study this structure. From ultrastructure perspective, it consists of three layers that are named from the superficial to the deeper one, lucid lamina (or pars rara), dense lamina (or basal lamina), fibrous-reticular lamina (or dense sublamina). Its main function is cellular adhesion, which is reached by anchoring fibers 6-11. Studies demonstrated that anchoring fibers emerge from the dense lamina to the superficial lamina of lamina propria, in which they chance direction to go back to dense lamina. Collagen III fibers of the lamina propria superficial layer run through the links formed by anchoring fibers, forming a chain that attaches firmly to the vocal fold epithelium of the lamina propria 12. As to chemical composition, electron immunohistochemical techniques demonstrate that the basement membrane comprises three main types of macromolecules: collagen IV, laminin and sulfate heparan proteoglycan. Collagen IV is responsible for strength and elasticity, whereas laminin and sulfate heparan proteoglycan are implied in cell adhesion and control of the passage of substances through the basement membrane. Fibronectin and entactin are glucoproteins also found in the basement membrane, but it is not known for sure what their function is. Collagen VII is also found on the basement membrane as the main component of anchoring fibers 6, 7.
Vocal fold mucosa can sometimes present lesions as a result of mechanical phonation trauma, as well as other types of aggression: physical, chemical and/or biological. Gray et al., by means of two similar studies, demonstrated histologically by electron microscopy the presence of acute phonation trauma. Using artificial methods, two groups of dogs were submitted to continuous phonation for two and four hours. In the first study, in the group submitted to phonation for two hours, they demonstrated destruction of microvilli of cell surface and increase in cell desquamation. In the group submitted to phonation for four hours, destruction of epithelium was more marked, with almost total cell desquamation. In the second study they also demonstrated presence of marked cell desquamation, but without impairment of epithelium germinating layer, basement membrane or lamina propria in the group submitted to two hours of phonation. After four hours of phonation, they observed greater epithelial destruction, presence of fluid among cells, destruction of organelles of remaining cells of cell surface. They also demonstrated the presence of fluid in the basal cells of the epithelium and basal membrane and between the membrane and lamina propria, owing to damage to anchoring fibers, caused by phonation trauma 13,14.
Damage resulting from phonation trauma, which can be characterized as inflammatory processes, may manifest in different forms. The most common ones are vocal nodule, polyp and laryngeal edema. The differentiation between them is many times difficult, normally labeled based on macroscopic aspect and clinical data. There is no histopathological picture defined by these inflammatory laryngeal lesions.
In the past, many authors had drawn attention to some characteristics of laryngeal inflammatory damage, as well as to the difficulty of histology diagnosis. Ash reported the presence of vocal fold damage resulting from trauma, which he called laryngeal node. Laryngeal node presented four progression stages according to macroscopic and histological findings: fibroid, polypoid, varicous and hyaline. According to the author, there was no precise limit between the four stages concerning the histology manifestations, not even when there were pathognomonic microscopic changes of each lesion. He also emphasized that the diagnosis could be made by an experienced laryngologist, through clinical findings 15. Fitz-Hugh et al. studied retrospectively three hundred samples of inflammatory laryngeal lesions, among which there were laryngeal nodules and polyps. Laryngeal nodule was considered the specialized response to vocal trauma, and the most common inflammatory laryngeal lesion. The author emphasized the difficulty to clinically and histologically differentiate laryngeal nodules from polyps. The microscopic aspect of the same lesion was assessed differently by different pathologists and what was classified as nodule by one, was classified as polyp by the other. The same difficulty was also faced by laryngologists, since clinically the lesions are similar, especially nodules and polyps. To sum up, he reinforced the importance of pathologists and laryngologists work together as a team for more precise diagnosis.16
Phonation trauma has been pointed as the main causing factor of inflammatory laryngeal lesions. Nagata et al. analyzed retrospectively over one thousand patients with diagnosis of vocal nodule or polyp. They concluded that the main etiological factor for vocal nodules and polyps is phonation trauma, and they did not find positive association between smoking and the referred lesions 17. Lancer et al., in his review on vocal nodules, addressed phonation trauma as the main etiology for the referred lesion. He emphasized the difficulty in differentiating vocal nodules and polyps, and also the fact that vocal nodules could be considered a subcategory of vocal polyps 18.
Recently, some authors have characterized both clinically and histologically the inflammatory laryngeal lesions. Hirano characterized vocal fold nodules as small white, sessile, bilateral symmetrical lesions located on the middle point of membranous vocal fold, anterior-posterior, on the free margin. As to histology, he reported edema and increase of collagen fibers on the lamina propria superficial layer. Vocal polyps presented greater diversity concerning color, size and shape. They may be uni or bilateral, frequently located on the median portion of the membranous vocal fold, on its free margin. Microscopically, alterations are located on the superficial layer of lamina propria and are characterized by intra-tissue bleeding, hyaline degeneration, thrombosis, edema, collagen fiber proliferation, and infiltration of inflammatory cells. In laryngeal edema or Reinke's edema, the membranous vocal fold presents edema in all its extension, with bilateral but asymmetrical impairment. The main affection found under microscopy is edema of lamina propria superficial layer 2, 5.
According to Pontes et al., nodules, polyps and edemas are vocal fold mucosa inflammatory abnormalities resulting from aggressive agents, among which the most important one is phonation trauma. Vocal nodule is the entity characterized by the association of three factors: laryngeal configuration, muscle tension and vocal abuse. The association of female or childish laryngeal pattern with muscle tension syndrome leads to posterior middle triangular chink. The vertex of this triangular chink is located in the transition from the anterior to the middle third of the free vocal fold margin, in which most of the vibration energy is placed during phonation. In this location, the vocal trauma leads to damage of nodular aspect in both vocal folds; therefore, the clinical entity is named vocal nodule. Macroscopically, nodules are characterized as rounded, sessile, white lesions whose appearance may range from edema to fibrosis. Microscopic abnormalities of vocal nodules are located on the most superficial layer of lamina propria and the epithelium, in which there is varied degree of edema and fibrosis, but without exuberant vascularization.19,20
Polyps, differently from nodules, present very different forms and consistencies, with varied degrees of vascularization, normally single, of varied location, but restricted to a small area of cord surface, being either sessile or pediculated. It is known that polyps are more vascularized lesions than nodules. Phonation trauma is one of the etiologies of vocal polyps, but differently from nodules, phonation trauma is not the most common etiology for this lesion. Irritating agents, allergy, and acute infections can contribute to the onset of polyps. As to microscopic aspects, it is observed that alterations are more deeply located on the surface of lamina propria when compared to vocal nodules. Epithelial changes are not very frequent 1, 3.
Laryngeal edema, also known as Reinke's edema, corresponds to extensive edema of vocal folds, bilateral in most cases, causing bulging of the glottis. The extensive aspect of the lesion is the main characteristic that distinguishes it from polyps. Vocal abuse is also responsible for the onset of the lesion, but it is not as important an etiology as vocal nodule, which is considered typical after vocal trauma. The histopathological picture presents affection primarily of lamina propria superficial layers (Reinke's space). There is increase in local vascularization, increase in extravascular fluid and presence of inflammatory cells in the site. 1-3,21
Despite those morphological and etiological differences, in many situations the clinical diagnosis of nodule, polyp and laryngeal edema is difficult or even impossible, requiring information concerning microscopic aspects. Therefore, even from the histological perspective, many times the differentiation among the referred lesions, as previously described, is hard to obtain and they are all labeled as non-specific inflammatory processes. Considering the different etiologies, we should adopt different clinical management approaches in each situation. Thus, it is necessary to find more elements that can ensure the correct diagnosis.
OBJECTIVE
Our purpose in the present study was to investigate, using histology and immunohistochemical techniques, the aspect of the vocal nodule epithelium considering two other laryngeal inflammatory lesions - polyp and laryngeal edema - as well as the correlations with vocal folds without macroscopic damage.
METHOD
The lesions studied in the present study - polyp, nodule and laryngeal edema - were obtained from medical chart analysis of patients seen by Instituto da Laringe from 1995 and 2001. The diagnosis of the damage was confirmed through review of videostroboscopic examination, whose images were stored in videocassette tapes.
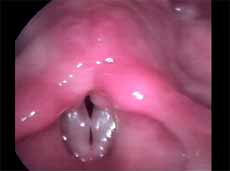
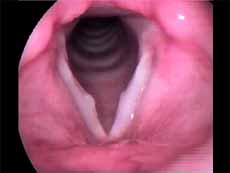
In this study we considered only nodules found and defined as vocal nodule clinical entity. We did not include nodules of other etiologies. Vocal nodules were characterized as rounded, sessile, white lesions, associated with medium-posterior triangular chink, located on the transition of the anterior to the medium third of the vocal folds, in the vertex of the triangular chink, bilateral, symmetrical or not, which moved with the mucosa wave during phonation (Figures 1 and 2).
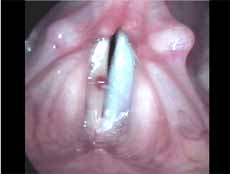
We characterized polyps as unilateral, sessile or pediculated lesions, which moved during phonation but not synchronous to mucosa wave, with jelly appearance and clinically presented by varied degrees of vascularization (Figures 3 and 4).
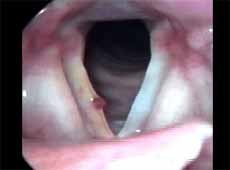
Laryngeal edema or Reinke's edema corresponded to diffuse edema that occupied the whole vocal fold and caused bulging of glottis, very mobile with phonation, bilateral and symmetrical or not (Figure 5).
The material comprised 26 inflammatory laryngeal lesions in 26 different patients. Mean age of patients was 36.5 years, ranging from 4 to 66 years. As to gender, there were 12 (46%) men and 14 (54%) women. Out of 26 lesions, 9 were vocal nodules, 8 were polyps and 9 were laryngeal edema. As to nodules, we analyzed randomly only one of the nodules. As part of the material, we included four vocal folds obtained from autopsy of four cadavers, without history of laryngeal disease or prolonged orotracheal intubation, which macroscopically did not present lesions. This group formed the control group and comprised 2 men and 2 women, aged between 15 and 60 years.
All patients had been submitted to surgery by the same ENT physician from 1995 to 2001. In the cases with vocal nodule, we indicated surgery for the cases that did not improve after three months of voice therapy.
After surgical removal, lesions were fixed in buffered liquid formalin (10%) and after 24 hours they were blocked in liquid paraffin. Paraffin blocks were obtained from the clinical pathology laboratory. As to the control group, excised larynges were fixed in buffered liquid formalin (10%) and the medium portion of vocal folds was dissected and blocked in liquid paraffin. Later, 5-micron sections were made and placed on glass slides. The sections were submitted to hematoxylin-eosin staining and Periodical Acid of Schiff (PAS) for microscopic analysis. Immunohistochemical reaction was made with streptoavidin-biotin-peroxidase technique 22. Three-micron sections were placed on glass slides and submitted to antibodies: antilaminin, anti-collagen type IV (Dako Corp., USA).
Readings were performed by the same pathologist, under optical microscopy. The study comprised only specimens that presented intact epithelium (cellular portion and basement membrane). In order to analyze morphological variables of epithelium, we used slides stained by hematoxylin-eosin and PAS techniques. Morphological aspects analyzed were: epithelial hyperplasia, keratosis, acanthosis, parakeratosis, dyskeratosis, dyskaryosis. We defined epithelial dysplasia as diffuse increase of epithelial thickness; keratosis as increase of superficial stratum thickness, with keratinization; acanthosis as increase in thickness of Malpighian stratum; parakeratosis as increase of superficial stratum thickness, with incomplete keratinization; dyskeratosis as isolated keratinization of epithelial cells, regardless of the stratum on which it was located; dyskaryosis as nuclear atypia with variation of diameter, irregularity of chromatin distribution and color abnormalities. We used semi-quantitative methodology assessing each item concerning its intensity and subjectively classifying them as:
Grade 0 or absent: absent abnormality
Grade 1: low intensity abnormality
Grade 2: moderate intensity abnormality
Grade 3: high intensity abnormality
Lesions classified as grade 3 presented more severe abnormalities compared to other lesions in the sample.
Both in sections stained by PAS techniques and submitted to immunohistochemical methodology, basement membrane was measured under optical microscopy with 10x ocular and 40x objective lens. The measures were made by placing a calibrated microscopic ruler with divisions of 0.01mm under the prepared material. Some of the samples were not identified by the three used techniques (PAS, laminin, collagen IV) owing to absence of enough material for analysis, but basement membrane identification by at least one of them was enough for study eligibility.
We calculated the mean of basement membrane thickness for each diagnosis, based on measures obtained in each one of the three techniques used.
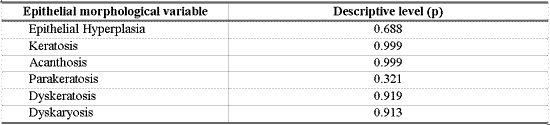
In order to check the association between morphological variables of epithelium with polyp, nodule and laryngeal edema we used the extension of Fisher exact test23. We considered as statistically significant difference values that were equal or below 0.10 ("p" below or equal to 0.10). Results that produced significant values were presented in bold.
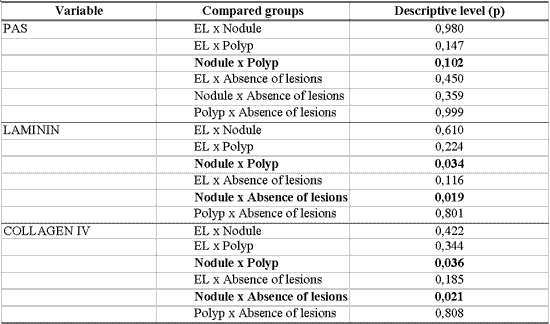
In order to check the association between inflammatory laryngeal lesions and vocal folds without macroscopic lesions with thickness of basement membrane stained by PAS and immunohistochemistry, we used variance analysis as fixed factor followed by multiple comparisons of Tukey method 24. We considered as statistically significant difference values that were equal or below 0.10 ("p" equal or below 0.10). Results that produced significant values were presented in bold.
RESULTS
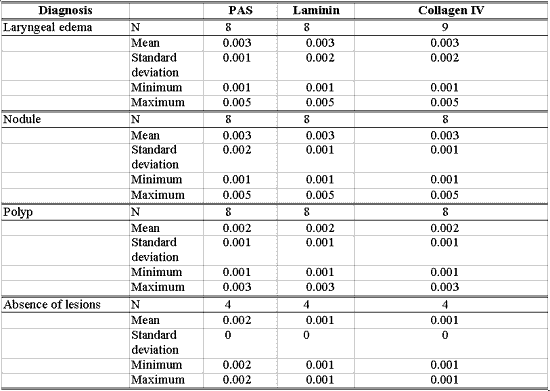
Tables 1 to 6 present a descriptive analysis of results concerning morphological variables of epithelium. Table 7 shows the descriptive analysis of data, relating measurement of basement membrane thickness using PAS staining technique and immunohistochemical detection with laminin and collagen IV, with different diagnoses.
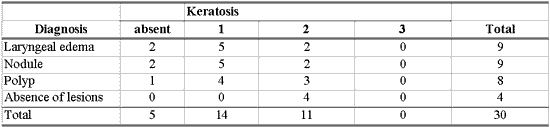
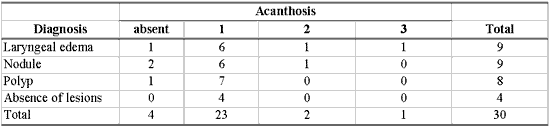
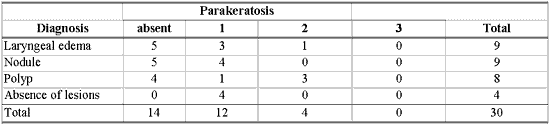
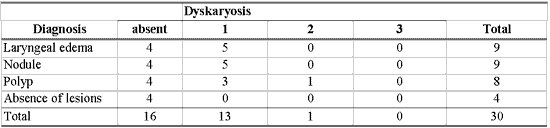
As shown in Table 8, there was no association between morphological variables of epithelium and inflammatory laryngeal damage, that is, it was not possible to differentiate nodule, polyp and edema based on morphological epithelial abnormalities (p> 0.10).
There was greater immunoexpression of laminin and collagen IV in basement membrane of nodules when compared to polyps (p=0.034 and p=0.036, respectively), as well as when compared to vocal folds without macroscopic lesions (p=0.019 and p=0.021, respectively). Nodules tend to present higher thickness in the basement membrane when using PAS staining in relation to polyps (p=0.102). Basement membrane in laryngeal edema was not differentiated from the others with PAS staining or with immunoexpression of laminin and collagen IV (Table 9).
DISCUSSION
One of the main functions of basement membrane is cellular adhesion, an important structure for the integrity of vocal fold epithelium. Out of all components, the ones that are the most related to cellular adhesion are collagen IV and laminin. Inflammatory laryngeal lesions are resultant from different types of trauma, so we expected to find a basement membranes that could be differentiated in each lesion. We decided to study collagen IV and laminin because these are the most important components in adhesion of epithelium and lamina propria 6, 7.
Basement membrane is a very small structure that has little definition under optical microscopy. In our study, we decided for this method because the purpose was to study microscopic alterations that characterized the histopathological picture of inflammatory laryngeal lesions, using methods and staining accessible to daily practice.
According to Hirano, one of the main principles of phonosurgery in laryngeal inflammatory lesions is the smallest manipulation possible of vocal folds so as to produce minimum wound. The main purpose is to preserve the lamina propria, especially its superficial layer, to avoid epithelial adherence among vocal folds. As our material resulted from microphonosurgery, we did not reach lamina propria to be studied, which made us restrict our analysis to the epithelium 5.
Once the analysis was made, we decided to correlate our findings with some aspects of the literature. In our sample, patients with diagnosis of vocal nodule were all children and women. Considering the concept of vocal nodule, we know that they are exceptionally found in men 19, 20. The literature points to higher incidence of polyps in men than in women, which was also observed by our study 17, 25.
Many different types of epithelial morphological abnormalities were observed in inflammatory laryngeal lesions in our study: hyperplasia, keratosis, acanthosis, parakeratosis, dyskeratosis and dyskaryosis. We could not, however, differentiate nodule, polyp and edematous cordites based on these abnormalities. Kambic et al. retrospectively studied samples of vocal fold polyp. Many types of epithelial abnormalities were observed, from severe atrophy to various degrees of hyperplasia, with or without keratosis. There was no pathognomonic epithelial abnormality in the vocal polyp. According to the authors, the distinction between nodule and polyp depended on personal experience of the pathologist, since the two lesions presented histological alterations that were quite similar 26. Loire et al. studied inflammatory laryngeal lesions through optical microscopy and found vocal nodules with various types of epithelial morphological abnormalities, among which the most common were: dyskeratosis (82%) and parakeratosis (66%). In vocal polyps and edematous cordites they observed epithelial abnormalities that were less common and less frequently found than in vocal nodules: atrophy or acanthosis of the epithelium in cases of polyps, or irregular thickness in cases of laryngeal edema 27. Kotby et al. observed through electron microscopy the presence of epithelial thickness in vocal nodules and polyps, more frequent in the former 25. Dikkers, Nikkels studied inflammatory laryngeal lesions through optical and electron microscopy and observed hyperkeratosis and parakeratosis of varied intensity. They concluded that isolated epithelial alterations are of no avail to the diagnosis because they cannot characterize inflammatory laryngeal lesions 28.
As referred before, Hirano introduced the concept of cover body in which he considered the epithelium as a thin vibration capsule capable of containing the vocal fold during phonation. Thickness of basement membrane may lead to impaired vibration of the epithelium, resulting in dysphonia 5.
In this study, vocal nodules basement membrane was thickened when compared to the membrane of polyps and normal vocal fold epithelium, studied by PAS staining. There was also immunoexpression of collagen IV and laminin in the basement membrane of vocal nodules when compared to the epithelium of polyps and normal vocal folds. Such findings demonstrated disorganization of basement membrane in the lesion that is considered a typical response to phonation trauma, evidenced by deposit of collagen IV and laminin, molecules responsible for cellular adhesion. Our study contributed to show the participation of laminin in thickening of basement membrane in nodules, which had not been shown in the literature before.
Many authors have already reported affections to the basement membrane in inflammatory laryngeal lesions. Loire et al., using optical microscopy, detected thickness of basement membrane in nodules, since in polyps there was thinning of the membrane 27. Kotby et al. analyzed samples of vocal polyps and nodules through electron microscopy. They reported disorganization of basement membrane both in nodules and in polyps, being more frequent in the first one 25. Gray et al. studied the ultrastructure of basement membrane in vocal nodules and demonstrated total disarrangement of membrane architecture 8. Later, Gray et al. emphasized the presence of basement membrane alterations in vocal nodules: duplication of basement membrane, disorganization of the architecture, higher immunoexpression of collagen IV 4. In vocal polyps, the main abnormalities seemed to be restricted to the lamina propria. Dikkers, Nikkels microscopically characterized vocal nodules as a combination of thickness of basement membrane and absence of hemorrhage or edema; polyps corresponded to a combination of recent bleeding signals without basement membrane affections 28. Gray et al. and Courey et al. studied inflammatory laryngeal lesions through optical microscopy and reported thickness of basement membrane in vocal nodules owing to greater deposit of collagen IV, but on the other hand, the membrane in vocal polyps presented thickness level close to normal 29, 30.
In the present study, laryngeal edema was not differentiated from other inflammatory lesions, not even in vocal folds without macroscopic lesions compared to basement membrane affections. Gray et al. concluded that laryngeal edema and polyp presented normal basement membrane, and microscopic affections are mainly located in the lamina propria 29. Dikkers, Nikkels characterized laryngeal edema under optical and electron microscopy as a combination of basement membrane thickness and presence of edema, signs of bleeding and thickness of submucous vessels 28. Courey et al. found basement membrane with few abnormalities in cases of laryngeal edema 30.
Clinical diagnosis of nodule and polyp, based on endoscopic examination, is sometimes very hard to be made, since not always the lesions are clearly presented from a conceptual perspective, as previously shown. According to our results, differentiation between these two lesions under optical microscopy is quite clear. Thus, considering our objective, we can state that if under optical microscopy we make use of PAS staining and immunohistochemistry for laminin and collagen IV to detect basement membrane, it will be possible to gather relevant information about the final diagnosis of lesions.
CONCLUSION
From the study of histological differentiation of vocal nodules and polyps and laryngeal edema, based on basement membrane abnormalities evidenced by staining with PAS technique and immunohistochemistry techniques to detect laminin and collagen IV, we could conclude that vocal nodule is differentiated from polyp in the three techniques used and from the epithelium of vocal folds without macroscopic lesions in immunoexpression of laminin and collagen IV. Laryngeal edema was not differentiated from the other lesions nor from normal vocal folds, when using the techniques described above in the study of basement membrane. In the cellular portion of the epithelium we did not find differences between the many clinical diagnoses.
REFERENCES
1. Hirano M. Structure of Vocal Fold in Normal and Disease States. Anatomical and Physical Studies. ASHA Rep 1981; 11: 11-30.
2. Hirano M. Surgical Anatomy and Physiology of the Vocal Folds. In: Gould WM, Sataloff RT, Spiegel JR, editors. Voice Surgery. St Louis: Mosby; 1993. p. 135-58.
3. Colton RH, Casper JK, Hirano M. Laryngeal Histopathology. In: Colton RH, Casper JK, Hirano M. Understanding Voice Problems. A Physiological Perspective for Diagnosis and Treatment. Baltimore: Wlliams & Wilkins; 1990. p. 51-72.
4. Gray SD, Hirano M, Sato K. Molecular and Cellular Structure of Vocal Fold Tissue. In: Titze JR, editor. Vocal Fold Physiology: Frontiers in Basic Science. San Diego: Singular Publishing Group; 1993. p. 1-35.
5. Hirano M. Phonosurgical Anatomy of the Larynx. In: Ford CN, Bless DM, editors. Phonosurgery: Assessment and Surgical Management of Voice Disorders. New York: Raven Press; 1991. p. 25-41.
6. Abrahamson DR. Recent Studies on the Structure and Pathology of Basement Membranes. J Pathol 1986; 149: 275-8.
7. Leblond CP, Inoue S. Structure, Composition, and Assembly of Basement Membrane. Am J Anat 1989; 185: 367-90.
8. Gray SD. Basement Membrane Zone Injury in Vocal Nodules. In: Gauffin J, Hammarberg B, editors. Vocal Fold Physiology. San Diego: Singular Publishing Group, Inc.; 1991. p. 21-7.
9. Dikkers FG, Hulstaert CE, Oosterbaan JA, Cervera-Paz FJ. Ultrastructural Changes of the Basement Membrane Zone in Benign Lesions of Vocal Folds. Acta Otolaryngol (Stockh) 1993; 113: 98-101.
10. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J. Cell Junctions, Cell Adhesion, and Extracellular Matrix. In: Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J, editors. Molecular Biology of the Cell. 3rd ed. New York: Garland; 1997. p. 949 - 1009.
11. Seung-Ho C, Hyung-Tae K, Min-Sik K, In-Ja L, Han-Jong P. Influence of Phonation on Basement Membrane Zone Recovery After Phonomicrosurgery: A Canine Model. Ann Otol Rhinol Laryngol 2000; 109: 658-66.
12. Gray SD, Pignatari SSN, Harding P. Morphologic Ultrastructure of Anchoring Fibers in Normal Vocal Fold Basement Membrane Zone. J Voice 1994; 8: 48-52.
13. Gray DS, Titze I, Lusk RP. Electron Microscopy of Hyperphonated Canine Vocal Cords. J Voice 1987; 1: 109-15.
14. Gray DS, Titze I. Histologic Investigation of Hyperphonated Canine Vocal Cords. Ann Otol Rhinol Laryngol 1988; 97: 381-8.
15. Ash JE, Schwartz L. The Laryngeal (vocal cord) node. Trans Amer Acad Ophtal Otolaryngol 1944; 48: 323-32.
16. Fitz-Hugh GS, Smith DE, Chiong AT. Pathology of Three Hundred Clinically Benign Lesions of the Vocal Cords. Laryngoscope 1958; 68: 855-75.
17. Nagata K, Shigejiro K, Yasumoto S, Maeda T, Kawasaki H, Hirano M. Vocal Fold Polyps and Nodules. A 10-year review of 1.156 patients. Auris Nasus Larynx 1983; 10 (suppl.): 27-35.
18. Lancer JM, Syder D, Jones AS, LE Boutillier A. Vocal Cord Nodules: a review. Clin Otolaryngol 1988; 13: 43-51.
19. Pontes P, Behlau M. Exame Laringológico. In: Pontes P, Behlau M, editores. Avaliação e Tratamento das disfonias. São Paulo: Lovise; 1995. p. 143-67.
20. Pontes P, Kyrillos L, Behlau M, De Biase N, Pontes A. Vocal Nodules and Laryngeal Morphology. J Voice 2002; 16: 408-14.
21. Sato K, Hirano M, Nakashima T. Electron Microscopic and Immunohistochemical Investigation of Reinke's Edema. Ann Otol Rhinol Laryngol 1999; 108: 1068-72.
22. Taylor CR, Cooper LR, Kurman RJ. Immunoperoxidase technique. Arch Pathol Lab Med 1978; 102: 113-21.
23. Agresti, A. Categorical data analysis. New York: Wiley Interscience; 1990.
24. Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4th ed. Boston: Irwin; 1996.
25. Kotby MN, Nassar AM, Seif EI, Helal EH, Saleh MM. Ultrastructural Features of Vocal Fold Nodules and Polyps. Acta Otolaryngol (Stockh) 1988; 105: 477-82.
26. Kambic V, Radsel Z, Zargi M, A?ko M. Vocal Cord Polyps: Incidence, Histology and Pathogenesis. J Laryngol Otol 1981; 95: 609-18.
27. Loire R, Bouchayer M, Cornut G, Bastian RW. Pathology of Benign Vocal Fold Lesions. Ear Nose Throat J 1988; 67: 357-62.
28. Dikkers FG, Nikkels PGJ. Benign Lesions of the Vocal Folds: Histopathology and Phonotrauma. Ann Otol Rhinol Laryngol 1995; 104: 698-703.
29. Gray SD, Hammond E, Hanson DF. Benign Pathologic Responses of the Larynx. Ann Otol Rhinol Laryngol 1995; 104: 13-8.
30. Courey MS, Scott MA, Shohet JA, Ossoff RH. Immunohistochemical characterization of Benign Laryngeal Lesions. Ann Otol Rhinol Laryngol 1996; 105: 525-31.
Figure 1. Vocal nodule. Vocal folds in adduction.
Figure 2. Vocal nodule. Vocal folds in abduction.
Figure 3. Angiomatous polyp. Vocal folds in adduction.
Figure 4. Angiomatous polyp. Vocal folds in abduction.
Figure 5. Edematous cordites. Vocal folds in abduction.Table 1 - Number of patients according to diagnosis and epithelial hyperplasia from 1 to 3.Table 2 - Number of patients according to diagnosis and grade of keratosis from 1 to 3.Table 3 - Number of patients according to diagnosis and grade of acanthosis from 1 to 3.Table 4 - Number of patients according to diagnosis and level of parakeratosis from 1 to 3.Table 5 - Number of patients according to diagnosis and grade of dyskeratosis from 1 to 3.Table 6 - Number of patients according to diagnosis and grade of dyskaryosis from 1 to 3.Table 7 - Number of patients with basement membrane identified by PAS and immunohistochemical methods in different diagnoses and data related to thickness obtained in millimeters.Table 8 - Results of the study between the association of diagnosis and epithelial morphological variable.
Fisher exact test: p < 0.10Table 9 - Analysis of the association between diagnosis and thickness of basement membrane in PAS staining and immunohistochemistry for laminin and collagen IV.
EL= Laryngeal edema
Analysis of variance: p< 0.10