Year: 2004 Vol. 70 Ed. 3 - (10º)
Artigo Original
Pages: 353 to 358
Sensitivity and specificity of middle latency potential
Author(s):
Eliane Schochat 1,
C. M. Rabelo 2,
R. C. De A. Loreti 3
Keywords: electrophisiology, evoked potentials, hearing tests, sensitivity, specificity
Abstract:
The Middle Latency Response (MLR) is a neuroelectrical response that can be recorded in the far field using scalp electrode. Nowadays it is suppose to be one of the best evoked potential for evaluate lesion or dysfunction of the central auditory nervous system. The MLR waves occur between 10 and 80 msec (ms) after the stimulus onset. The Pa is the most reliable because it is the most visible and robust of this potential. Because of the big variability of the amplitude and the latency of the MLR waves it is not yet largely used in the clinical set. Aim: The purpose of this study was to establish the sensitivity and specificity of the MLR waves. Study design: Case-control. Material and Method: Individuals between 15 and 55 years old with lesion of the central auditory nervous system, with auditory processing disorders and normal one (control group) were evaluated using the MLR. Results: The results showed that the amplitude difference of 30% held the better results, for the electrode as well as the ear effect. The ear effect was more efficient in detecting auditory processing disorder while the electrode effect was better for detecting the lesion.
![]()
INTRODUCTION
Middle Latency Response (MLR) is a series of waves/neuroelectrical responses that can be recorded from the brain through surface electrodes.
The potential was first described by Geisler, Frishkopf, Rosenblith in 19581, but owing to absence of data that could confirm its effectiveness, the potential was attributed to a myogenic artifact, hindering and preventing its acceptance as a clinical procedure or hearing test. Fortunately this is no longer the case; the understanding about the procedure, recording techniques, involved anatomical structures that trigger it, influence of sleep and maturation made Middle Latency Responses become more accepted both clinically and for research purposes.
Currently, Middle Latency Response is seen as one of the most promising electrophysiological potentials (objective assessment method) to assess dysfunctions and/or abnormalities of the central auditory nervous system (CANS).
Middle latency potential is an electrophysiological test that has a series of waves that occur between 10 and 80 ms after the onset of sound stimulus 2.
Considering the potential value of revealing under-threshold brain dysfunctions and audiological findings, these tests can be used to confirm or even study these conditions. This technology can significantly increase current knowledge on auditory processing disorders, assisting and transforming clinical procedures in neurodiagnosis, collaborating to the understanding of plasticity of the central auditory nervous system 3, 4.
Electrophysiological responses do not depend on linguistic skills of subjects and except for late potentials they do not demand cognitive processing of sound stimulus.
In general, Middle Latency Response is characterized by two primary waves that tend to be wider, larger and of lower fundamental frequency than brainstem auditory responses. Pa wave is usually the most robust wave of middle latency, comparable to wave V at ABR 5.
Middle latency response seems to have many different generators, counting on the contribution of thalamus-cortical structures and less on the inferior colliculus and reticular formation. Studies have also shown that temporal lobe auditory reception contributes to middle latency responses, especially to Pa 2.
The amplitude of peaks Na and Pa is apparently bilaterally symmetrical with electrodes placed in a slightly posterior position to C3 and C4 6.
Since childhood and up to adolescence, detectability of wave Pa recorded during sleep increases, ranging from 20% in childhood (1 to 6 months) to 90% at the age of 12 years. This increasing tendency as a result of age stems from maturation; therefore, it is only possible to record these waves if the child develops normally, and it is not possible to do it if the child has neurological, cognitive or speech and language disorders.
Arehole, Augustine and Shimhadri in 19967 studied Middle Latency Responses with learning disorders (N=11) and without this disorder (N=11) in children aged 8 to 12 years with normal peripheral hearing and they found that mean latency of Pa component in Middle Latency Response obtained in children with learning disorder was significantly higher than the mean obtained for normal children in contralateral presentation. They also found greater variability in latency of wave Pa for the group with learning disorder.
Behavioral tests normally reveal functional deficits of auditory processing. Contrarily, electrophysiological tests reveal integrity and capability of the central auditory nervous system, and can confirm the level of site of lesion 8. Undoubtedly, electrophysiological tests can be more sensitive to some lesions in the CANS than any behavioral test 9.
However, clinically, it should be taken into account the fact that there is great variability in results found in inter-subjects middle latency potentials, which makes it difficult to define normal and/or abnormal measures. Middle latency topography in normal subjects is symmetrical, that is, electrodes placed on the right and left temporal lobes should produce similar responses. Subjects with lesion in one of the hemispheres should present reduced ipsilateral response on the affected side - the electrode effect 10, 11.
There may also be ear effect when the ear has significantly lower amplitude than the other in many electrode positions. This effect can be ipsi or contralateral to the lesion site 10-12.
So that this potential can be used both clinically and for research purposes it is necessary to define effectiveness-sensitivity and specificity criteria.
To that end, we studied individuals that had damage to central auditory nervous system confirmed by imaging tests or that had been submitted to cranial-encephalic surgeries, subjects with auditory processing disorders, confirmed by behavioral assessments and the results were compared with normal subjects of the same age range.
MATERIAL AND METHOD
The study was submitted to the Ethics Committee for Research Projects, Hospital das Clinicas, Medical School, University of Sao Paulo (HC-FMUSP), and it was approved on 14/06/2000 (protocol nº 288/00).
The subjects were aged 15 to 55 years, had damage of the central auditory nervous system, confirmed by imaging diagnosis, or had been submitted to cranial-encephalic surgery; subjects with auditory processing disorders (CAPD), and normal subjects (control group), of the same age range. All participants (or parents) signed the Free Informed Consent Term before starting data collection.
The criterion used for defining normal subjects in behavioral test was dichotic digit test, since it is easy to apply, quick and has good sensitivity and specificity 13.
We assessed 54 subjects being that 10 had CANS damage, 17 had CAPD and 27 were normal, all of them within the same age range.
We conducted the following assessments: immittanciometry, pure tone audiometry, speech reception threshold (SRT), speech percentage recognition index (SPRI), according to traditional method, to ensure integrity of the peripheral system, being that after it the electrophysiological test was performed with Middle Latency Response, being that it was recorded after ABR, using equipment brand Nicolet model Spirit. Brainstem integrity had to be ensured because disorders at this point or in the peripheral auditory system can contaminate the results of Middle Latency Response.
The test was conducted in silent environment and stimuli were presented in monoaural modality, at a speed of 9.8 clicks per second and intensity of 70dB normal hearing level nHL. The number of sweeps was 1,000 clicks and the recording window used was 72 ms.
We used low pass filter of 20Hz, since it is the best one to determine latency of waves and high pass filter of 1,500Hz2, with range of 12dB/octave, which allows recording of responses of Auditory Brainstem Response with Middle Latency Responses. After the analysis of ABR waves, they were digitally filtered, low pass 20Hz and high pass 200Hz, aiming at improving morphology of waves of Middle Latency, reducing the noise of recordings.
Electrodes were placed on the mastoids (A1 and A2), left, right temporal lobes or coronal region, and vertex (C3, C4 and Cz, respectively) and on the forehead (A - grounding electrode).
Before placing the electrodes, the areas where they were going to be fixed were cleaned in order to reduce electrical impedance between the skin and the electrode to at least 5 omhs.
Stimuli were sent by earphone and responses were recorded twice in each condition (C3A1, C4A1, CzA1; C3A2, C4A2 and CzA2) to increase reliability.
We measured latency and amplitude of wave Pa, since it was more robust; latency was measured in the wave peak and amplitude was measured using Na wave as base (previous wave) 5.
In the analysis of this test, we compared the tracings obtained with some common condition (ear or electrode), that is, each tracing compared to other two. C3A1 was compared to C3A2 (common electrode) and C4A1 (common ear), for example.
As the value used in the analysis of wave Pa amplitude, we considered three different range analyses, 30%, 40% and 50% of difference between the amplitudes of wave Pa in relation to the same electrode but on the other ear, that is, amplitude of wave Pa in condition C3A1 relative to condition C3A2, and on the same ear, but varying electrodes, that is, wave Pa amplitude in condition C3A1 in relation to condition C4A1 14.
The level of significance used in the test was 5%.
RESULTS
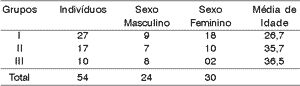
We assessed 54 subjects being that 27 (50%) were normal subjects (group I), 17 (31.5%) had auditory processing disorder (group II) and 10 (18.5%) had some central auditory nervous system damage (group III). There were 24 (44.44%) male and 30 (55.6%) female subjects. Mean age of assessed subjects was 31 years. Mean age of normal subjects was 26.7 years, for subjects with CAPD it was 35.7 years, and for subjects with neurological affection it was 36.5 years, as shown in Table 1.
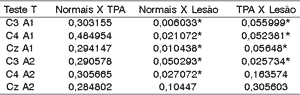
To assess whether there was significant difference between latency of wave Pa in middle latency potential among the groups, we applied T test, whose null hypothesis (Ho) was that normal subjects were equal to those with CAPD or CANS damage, that subjects with damage and CAPD were also equal and the alternative hypothesis (h1) that normal CAPD, and Normal damage, and finally subjects with damage CAPD. The level of statistical significance was 0.05.
As we noticed, there was significant difference in basically all latencies of wave Pa between the normal group and the group with damage (except for position CzA2). For CAPD and damage groups there was statistically significant difference in all positions related to left ear and only one position of the right ear - C3A2.
We did not detect statistically significant differences in Normal x CAPD groups.
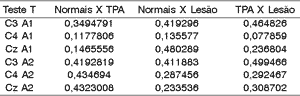
To assess the significant difference between amplitude of wave Pa in middle latency potential between the groups we applied T Test, whose null hypothesis (ho) was that normal subjects would be equal to those with CAPD and damage, that subjects with damage and CAPD would also be equal, and as alternative hypothesis (H1), that normal CAPD, and Normal Damage, and finally, subjects with Damage CAPD. The level of significance we used was 0.05.
As we can observe, there is no statistically significant difference between the 3 groups assessed concerning amplitudes of wave Pa.
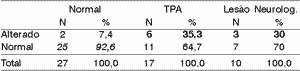
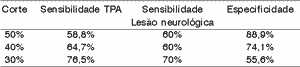
We can see the results of sensitivity and specificity for each difference of 50%, 40% and 30%. To each range of difference, we showed in bold sensitivity and in italics, specificity.
The difference range 30% was the best balance between sensitivity and specificity, even though it was not good criterion to identify the groups.
The difference ranges 30% and 40% were those that have the best balance between sensitivity and specificity, even though they were not very good criteria to identify the group.
In general, the ear effect produced better results than the electrode effect.Table 1. Distribution of subjects by gender and age range.
Table 2. T test applied to latency of Wave Pa among the 3 groups.
CAPD = central auditory processing disorder.
Table 3. T test applied to amplitude of Wave Pa among the 3 groups.
CAPD = central auditory processing disorder.
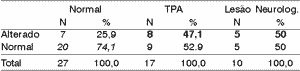
Table 4. Electrode effect - 50% range.
CAPD = central auditory processing disorder.
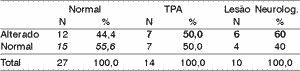
Table 5. Electrode effect - 40% range.
CAPD = central auditory processing disorder.
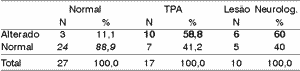
Table 6. Electrode effect - 30% range.
CAPD = central auditory processing disorder.
Table 7. General results - electrode effect.
CAPD = central auditory processing disorder.
Table 8. Ear effect - 50% range.
CAPD = central auditory processing disorder.
Table 9. Ear effect - 40% range.
CAPD = central auditory processing disorder.
Table 10. Ear effect - 30% range.
CAPD = central auditory processing disorder.
Table 11. General results - ear effect.
CAPD = central auditory processing disorder.
DISCUSSION
All analyses were conducted with wave Pa, since according to Kraus, Kileny and McGee, 19942 it seems that it is the most reliable wave to Middle Latency Potential, in addition to being generated in the auditory reception area, that is, in the temporal lobe. Hall in 19925 also stated that wave Pa was usually the most robust wave from Middle Latency and, thus, it is comparable to wave V of ABR. We can add that this potential seems to have many generators, counting on greater contribution from thalamus-cortical structures and less from inferior colliculus and reticular formation.
The intention to assess sensitivity and specificity of Middle Latency Responses was due to the fact that these potentials measure electrical activities of the brain and can be used to assess high-level cognitive processes. They are considered useful to assess specific aspects of information process and can, consequently, provide ideas about synchronism, ordering and interaction of auditory processes. For this reason, evoked potentials are used to study cognition present in brain mechanisms and to characterize the processing of information in healthy and impaired populations 15.
Middle latency measures that are more important to clinical practice are perfectly known and many studies have demonstrated the diagnostic value of this potential to assess CANS damage, but little is known about the diagnostic value of middle latency potentials in cases of CAPD, let alone the effectiveness of this important potential for the impairment of CANS.
Studies conducted by Jerger et al. in 200216 showed difference in results of electrophysiological tests of subjects with CAPD compared to normal subjects, being that the author attributed this difference to a deficit of efficiency in inter-hemispheric transfer of auditory information. Moreover, we have to consider that subjects with auditory processing disorders present neuromorphological abnormalities 17.
The study analyzed people with auditory processing disorders and confirmed CANS damage diagnosed by behavioral and/or imaging tests and we can say that the hit rate was average, meaning that not all subjects with CAPD and/or lesions were detected by electrophysiological assessment. These data are in accordance with the reports by Arehole, Augustine and Shimhadri (1996)7 which studied Middle Latency Responses of children with and without learning disorders, ages ranging from 8 to 12 years, with normal peripheral loss, and found greater variability of latency in wave Pa in the group with learning disability; however, it was not statistically significant difference.
It is important to notice that the potential used to study these latency measures are normal even in subjects with cortical lesions and these data were also found by studies with subjects who had unilateral cortical lesions, which was the case of many studied subjects 18.
There was no affection to latencies of CAPD group subjects. It is in agreement with previous studies, such as the ones by Jirsa in 200119 which showed there was no significant change between latency of waves in electrophysiological tests and normal subjects and those with CAPD.
It is important to notice that in both studies previously reported 18, 19, amplitudes were normally reduced.
However, a key point in determining diagnosis using middle latency that seems to be a consensus between the many researchers is that variability of inter-subject amplitude is very wide. Given that it was also found in the present study, according to what is seen in Table 3, what is used is the comparison between the many electrodes placed, that is, intra-subject variability.
We still need studies concerning reliability of the potential to identify and diagnose abnormalities of the central auditory nervous system as proposed by Kileny, Paccioretti, Wilson, 198720; Ibanez Deiber, Fischer, 198921; Kraus, McGee and Comperatore, 198922; Shehata-Dieler et al, 199123; Musiek and Lee, 199712, but all of them agree that auditory evoked middle latency potential is quite promising.
According to the variation range used in this study, we can see (Tables 4 to 11) that the 30% range is the one that has the best results, both for electrode effect and ear effect and that ear effect is better than electrode effect.
It is also possible to notice that ear effect is more reliable to prevent auditory processing disorders, whereas electrode effect is more effective to evidence damage.
CONCLUSIONS
As a result of the present study, we concluded that 30% range is the most reliable band to identify damage to central auditory system and auditory processing disorders. Even though middle latency auditory evoked potentials are a promising test to show integrity and maturation of central auditory system, issues such as the effect of filters and their relation with assessment in the general population still require further investigation.
REFERENCES
1. Geisler C, Frishkopf L, Rosenblith W. Extra cranial responses to acoustic clicks in man. Science 1958; 128: 1210-1.
2. Kraus N, Kileny P, McGee TJ. Middle latency auditory evoked potentials. In: Katz, J. (ed). Handbook of clinical audiology, Baltimore: Williams & Wilkins; 1994.
3. Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol 1990; 47(8):919-26.
4. Jirsa, RE. The clinical utility of the P300 AERP in children with auditory processing disorders. J Speech Hear Res 1992; 35: 903-12.
5. Hall III, JW. Handbook of Auditory Evoked Responses. Boston: Allyn and Bacon; 1992.
6. Musiek, FE. Auditory evoked responses in site of lesion assessment. In: Rintelmann WF (ed) Hearing Assessment, 2a Ed. Boston: Allyn & Bacon; 1991.
7. Arehole S, Augustine L, Shimhadri R. Middle latency response in children with learning disabilities: Preliminary findings. J Communications Dis 1996; 28: 21-38.
8. Musiek, FE, Gollegly KM. Maturational considerations in the neuroauditory evaluation of children. In: Bess, FH. Hearing Impairment in Children. Parkton: York Press; 1988. p. 231-50.
9. Baran JA, Musiek FE. Behavioral assessment of the central auditory nervous system. In: Rintelmann WF (ed). Hearing Assessment. 2nd Ed. Boston: Allyn & Bacon; 1991.
10. Musiek FE, Baran JA, Pinheiro M. Neuroaudiology Case Studies. San Diego: Singular Publishing Group; 1994.
11. Kraus N, McGee TJ. The middle latency response generating system. Electroencephalography and Clinical Neurophysiology 1995; 44 (Suppl.): 93-101.
12. Musiek FE, Lee W. Conventional and maximum length sequences middle latency response in patients with central nervous system lesions. J Am Acad Audiol 1997; 8: 173-80.
13. Musiek FE. Assessment of central auditory dysfunction: The Dichotic Digit Test revisited. Ear Hear 1983; 4: 79-83.
14. Musiek FE, Charette L, Kelly T, Lee W, Musiek E. Hit and False Positive Rates for Middle Latency Response in Patients with Central Nervous System Involvement. J Am Acad Audiol 1999; 10 (3): 124-32.
15. Konishi T, Naganuma Y, Hongou K, Murakami M, Yamatani M, Yagi S. Changes of P300 latency with age in childhood epilepsy. Pediatr Neurol 1995; 12:132-5.
16. Jerger J, Thibodeau L, Martin J, Mehta J, Tillman G, Greenwald R et al. Behavioral and Electrophysiologic Evidence of Auditory Processing Disorder: a Twin Study. J Am Acad Audiol 2002; 13 (8): 438-60.
17. Chermak GD. Deciphering auditory processing disorders in children. Otolaryngol Clin North Am 2002; 35(4):733-49.
18. Kraus N. Speech sound perception, neurophysiology, and plasticity. International Journal of Pediatric Otorhinolaryngology 1999; 47(2):123-9.
19. Jirsa, RE. Maximum Length Sequences. Auditory Brainstem Responses from Children with Auditory Processing Disorders. J Am Acad Audiol 2001; 12(3): 155-164.
20. Kileny P, Paccioretti D, Wilson AF. Effects of cortical lesions on middle-latency auditory evoked responses (MLR). Electroencephalography & Clinical Neurophysiology 1987, 66(2):108-20.
21. Ibanez V, Deiber P, Fischer C. Middle latency auditory evoked potentials and cortical lesions: criteria of interhemispheric asymmetry. Arch Neurol 1989; 46: 1325-32.
22. Kraus N, McGee TJ, Comperatore C. MLR's in children are consistently present during wakefulness, Stage I and REM sleep. Ear Hear 1989; 10: 339-45.
23. Shehata-Dieler W, Shimizu H, Soliman S, Tusa R. Middle latency auditory evoked potentials in temporal lobe disorders. Ear Hear 1991; 12: 377-88.
1 Full Professor, Speech Pathology and Audiology, FMUSP.
2 Speech pathologist and Audiologist. University of Sao Paulo. Master studies under course, Program of Experimental Pathophysiology, FMUSP.
3 Speech pathologist and Audiologist. University of Sao Paulo Master studies under course, Program of Experimental Pathophysiology, FMUSP.
Address correspondence to: Eliane Schochat - Rua Baronesa de Itu, 788 ap. 61 Higienópolis 01231-001 Sao Paulo SP
Tel: (55 11) 3826-8358 / 99994266 - E-mail: eschocha@usp.br
Study conducted at the Course of Speech Pathology and Audiology, FMUSP. Financially supported by Fundação de Amparo e Pesquisa do Estado de Sao Paulo - FAPESP.