Year: 2002 Vol. 68 Ed. 6 - (15º)
Artigos Originais
Pages: 874 to 881
Transient evoked otoacoustic emissions analysis in infants with and without hearing loss risk indicators
Author(s):
Priscila Denzin 1,
Renata M. M. Carvalho 2,
Carla G. Matas 2
Keywords: otoacoustic emissions, neonates with risk indicators, hearing evaluation
Abstract:
Aim: This study aimed to verify the transient otoacoustic emissions responses in infants with and without risk indicators for hearing loss. Study design: Clinical prospective. Material and method: A sample of 44 infants participated of the study: 22 infants post-conceptual aged between 08 and 65 days of life with one or more risk indicators, and 22 infants with post-conceptual ages between 07 and 30 days of life with no risk indicators. All of them were born on University Hospital of the São Paulo University. Results: The performance on otoacoustic emissions was based on parameters of reproducibility of A-B curves, signal/noise amplitude by frequency bands and response for each ear tested. Conclusion: We concluded that infants with risk indicators presented poorer performance in comparison with no risk infants.
![]()
INTRODUCTION
The detection of auditory abnormalities of cochlear origin using evoked otoacoustic emissions consists in a relatively simple, quick, non-invasive method that can be used at any age range; this paper emphasizes its application in infants.
The phenomenon of sound production from the cochlea is related to cochlear micromechanics process, suggesting the existence of a mechanically active component, coupled to the basilar membrane, through which there is the reserve process of sound energy transduction. This property is attributed to outer hair cells and controlled through efferent auditory pathways (Oliveira, 1993; Lopes Filho & Carlos, 1996). Therefore, otoacoustic emissions are also important during central audiological assessments because they enable differential diagnosis of cochlear and retrocochlear pathologies through the use of contralateral and ipsilateral supression (Denzin, 1999; Eckley, 1996; Hood, 1993; Durante & Carvallo, 2001).
Otoacoustic emissions can be classified into: spontaneous (measured in the absence of acoustic stimulation) and evoked, which are subdivided into transient, stimulus-frequency and distortion product (Kemp, 1978).
In our study, we used transient otoacoustic emissions since it is a quicker method that consists of probe positioning (that contains the stimulus generator and a microphone) at the entry of the external acoustic canal. The sound stimulus (click) passes though the middle ear and cochlea, and if the functions are preserved, it will produce an echo backwards, to be recorded by the microphone on the external auditory canal; we should bear in mind that this method does not quantify hearing loss, only detects its presence (Kemp et al., 1986; Probst et al., 1991).
The prevalence of hearing loss in apparently normal neonates is estimated in 1:1,000, but it grows dramatically to 1:50 in high-risk newborns (Mhel & Thomson, 1998). Early diagnosis in children is recommendable, preferably in the first 6 months of life, considering the irreversible abnormalities of language acquisition, development processes and cognitive skills that can result from impairment detected only years later.
Neonates have the best pre-requisites for the test because they are easily accessible, in addition to being inactive and quiet for long periods of time. Thus, we focused our study in the analysis of transient otoacoustic emissions with and without hearing loss risk indicators, according to the “Joint Committee on Infant Hearing” (1994) and we used the theoretical evidence presented by the authors that follow.
Kemp (1978) recorded otoacoustic emissions in the external auditory canal and classified them basically into two types: spontaneous, that can be recorded in the external auditory canal without acoustic stimulus, and evoked, that result from the presentation of a sound stimulus. He also stated that otoacoustic emissions are energy produced at the cochlea that can be released spontaneously or as consequence to a sound stimulus.
Previc (1991) described a theory whose basic principle suggested that cerebral lateralization in humans was a consequence of prenatal asymmetry of the ear and labyrinth. Such asymmetry would be manifested by a slight advantage in right monaural sensitivity, presumably resultant from greater middle ear conduction. Thus, the origin of the right ear advantage would be related to greater monaural sensitivity on the right for sounds between 1,000 and 6,000Hz.
Probst et al. (1991) stated that, concerning specifically the middle ear, otoacoustic emissions can be recorded by the external auditory canal if vibration energy can be conducted reversibly, from the cochlea through the ossicle chain to the tympanic membrane, where it is transformed into acoustic signal.
Kok et al. (1992), in a study with 1,036 ears of healthy newborns, assessed between 3 and 238 hours after birth, in a silent room, recorded a mean amplitude of 21.9 dB SPL and greater reproducibility in older newborns.
Oliveira (1993) related the origin of otoacoustic emissions to the active mechanisms of the cochlea, specifically those related to outer hair cells, which even though do not operate as cochlear receptors because they do not codify the sound message, they are able to have both fast and slow contractions, working as active cochlear effectors since they release mechanic energy during the fast contractions and become responsible for the otoacoustic emissions.
Smurzyinski et al. (1993) tested two groups of term and preterm newborns and noticed mean amplitude of 19.0dB SPL in the term group and reported that the amplitude values for the pre-term group with hearing loss risk was lower than the term group values.
The “Joint Committee on Infant Hearing” (1994) published the indicators of sensorineural or conductive hearing loss risk in newborns when universal screening is not available: family history of congenital or late onset sensorineural hearing loss; congenital infections associated with sensorineural hearing loss or suspected of being associated, such as: toxoplasmosis, syphilis, rubella, cytomegalovirus and herpes; craniofacial anomalies including morphological abnormalities of the pinna and external auditory canal; weight at birth below 1,500g; hyperbilirubinemia with levels that exceeded the indication of exsanguineous transfusion; ototoxic medication including but not limited to aminoglycosides used in combination with loop diuretics; bacterial meningitis; severe anoxia at birth including Apgar of 0 to 4 in the first minute and 0 to 6 in the fifth minute; mechanical ventilation for five or more days; signals or other findings associated with syndromes that can be related to sensorineural hearing loss.
Aidan, Lucek, El-Bez, Lestang, Parrat, Pascu, Avan & Bonfils (1995), when conducting a study with 254 term newborns, without complications, noticed that the mean general amplitude of transient otoacoustic emissions on the right (21.05 dB SPL) was statistically superior to that on the left (19.54 dB SPL).
Silva et al. (1995) studied the etiology of deafness in 1,160 patients for four years and had the following frequency of occurrence: undetermined (30%), rubella (21%); birth anoxia (12%); prematurity (8%); ototoxic drugs (10%), genetic causes (6%); hyperbilirubinemia (0.8%), meningitis (9%), others (3.2%). They concluded that a large proportion of the hearing loss cases is still correlated with perinatal affections justifying the importance of early detection programs.
Lopes Filho & Carlos (1996) described the most recent theories relating the origin of otoacoustic emissions and the active cochlear mechanisms, especially those related to the outer hair cells. They defined otoacoustic emissions as being the energy response in audiofrequency of the cochlea originated from the outer hair cells that can be recorded by a small microphone in the external auditory canal as a response to a click stimulus. For the otoacoustic emissions to be recorded in the canal it is essential that the tympanic ossicle system be absolutely normal, that is, any abnormalities in the middle ear can cause amplitude affections in the responses.
Aidan et al. (1997), in a study with 582 newborns with hearing risk noticed that the mean amplitude of transient otoacoustic emissions were 21.75 dB SPL. As to prevalence of ear, they showed statistically significant differences between the right (22.4 dB SPL) and left ears (21 dB SPL). They also detected a statistically significant difference between male (21.4 dB SPL) and female (22.1 dB SPL) patients.
Newmark et al. (1997) applied transient otoacoustic emissions in 120 term newborns and observed, among other things, general amplitude significantly greater on the right ear for both genders. According to the authors, these interaural differences suggest asymmetry in efferent cochlear inhibition.
Basseto et al. (1998) discussed the risk indicators for hearing loss proposed by the Joint Committee on Infant Hearing (1994), and pointed out that newborns that present one or more risk indicators should be screened before hospital discharge and maximum up to the third month of life. They also stated that the screening program depends on efficacy of procedures. They suggested that evoked otoacoustic emissions, auditory brainstem evoked potential and behavioral observation audiometry should be conducted, since the three methods explore different forms of auditory reaction.
Costa & Costa Filho (1998) reported that a large portion of the preterm newborns tend to present significant clinical complications, in addition to being susceptible to hypoxia episodes. Upon analyzing 36 pre-term newborns (37 to 44 weeks of conceptual age), using evoked otoacoustic emissions, they did not find statistically significant differences between right and left ears or genders, considering the results of reproducibility of waves A and B and responses by frequency of intensity. They noticed that cochlear responses in pre-term newborns have the highest values between the frequencies of 3,000 and 5,000Hz.
Mehl & Thomson (1998), in a study involving 41,796 children in a two-year period, using as tests transient otoacoustic emissions and auditory brainstem responses, had a frequency of bilateral congenital hearing loss of 1 to 500 births, and screening sensitivity of approximately 100%. They concluded, thus, that universal hearing screening is beneficial and justified by the frequency of the disease, the precision of the screening tests, the possibility of providing early intervention and the better results attributed to early amplification to prevent the costs of future interventions. Moreover, the incidence of bilateral congenital hearing loss is many times greater than the combined incidence of all tests conducted in blood samples of newborns.
Morlet et al. (1998) studied 1,531 risk neonates using transient otoacoustic emissions and auditory brainstem evoked potentials (in case of suspicion of hearing loss ). In the results, 88.9% of the newborns were normal and bilateral losses were detected in 58 cases, whereas unilateral losses were present in 26 cases.
Soares et al. (1998) studied the response pattern in newborns with and without auditory risk with otoacoustic emissions, behavioral observation and acoustic immittance measures and compared the results; they found 100% compatibility among the three screening procedures in the group of newborns without risk. In the group with hearing risk, they identified two children with moderate sensorineural loss. They also observed mean amplitude of 18.5 dB SPL and mean reproducibility of 86.4% in the risk group.
Paludetti et al. (1999), upon conducting universal hearing screening in newborns with transient otoacoustic emissions and auditory brainstem evoked potentials (in cases that failed the screening), obtained amplitude results of 21.49 dB SPL on the right and 21.78 dB SPL on the left for otoacoustic emissions, in which 77.2% of the tested ears passed the first test and 22.8% failed it. They concluded that the transient otoacoustic emissions test is the preferred method for universal screening, but they emphasized the importance of having further standardization of criteria, execution and result evaluation.
Rhee et al. (1999), concerning topodiagnosis of auditory lesion and test of reliability of transient otoacoustic emissions, conducted a study with 11 neonates submitted to total blood transfusion because of hyperbilirubinemia. They used audiological assessment with transient otoacoustic emissions and auditory brainstem evoked potentials. They observed in the results that babies passed otoacoustic emissions, but there were four abnormal results or absence of ABR responses. They concluded that the results indicated that the lesion site affected by hyperbilirubinemia could be the retrocochlear region, whereas the cochlea remains intact; otoacoustic emissions can have limitations in the assessment of hearing of neonates with hyperbilirubinemia.
Vallejo et al. (1999) conducted a study with newborns with and without auditory risks below the age of 12 months and noticed that the amplitude of transient otoacoustic emissions was smaller in the hearing risk group and the presence of emissions was more frequent in the group with no hearing risk. They had mean amplitude of 16.8 dB SPL in the risk group and mean amplitude of 21.6 dB SPL in the group without risk. They concluded, thus, that auditory risk influenced the incidence of presentations and amplitude of transient otoacoustic emissions.
MATERIAL AND METHOD
The present study was conducted at the Center for Education and Research of the University of São Paulo (CDP-USP), from September to December 2000, approved by the Research Ethics Committee of the Department under protocol number 202/02.
1. Material
There were two groups in the study: the first one comprised 22 infants with one or more hearing risk indicators, born at the University Hospital, University of São Paulo, with post-conceptual age ranging from 08 to 65 days, being 9 female and 13 male subjects; the second group consisted of 22 infants without hearing risk indicators born at the University Hospital, University of São Paulo, with post-conceptual age ranging from 07 to 30 days, being 13 female and 09 male subjects.
Owing to the long period of hospitalization and waiting for the infants to have better health status among those born with risk indicators, the hearing assessment with otoacoustic emissions was conducted at 28 days of post-gestational life, reason why we started to refer to the newborns as infants (29 days to 2 years) (Matas, 1998).
The inclusion criteria were: absence of neurological impairment, absence of tympanic-ossicle abnormalities, to have or not at least one risk indicator for hearing loss (Joint Committee on Infant Hearing, 1994).
The participation was voluntary and confirmed by parent’s signature in the Free Informed Consent Term.
2. Equipment
To conduct transient otoacoustic emissions, we used the device ILO88-4.2 OAE Analyzer System Otodynamic, connected to a microcomputer PC-486 and probe for newborns with sterilized tip for sealing of external auditory canal. We considered the following parameters: overall amplitude of responses, reproducibility of waves A and B, probe stability and amplitude of responses in frequencies 0.8Hz, 1.6KHz, 2.4KHz, 3.2KHz and 4.0KHz on the right ear and left ear separately.
3. Method
Study of Transient Otoacoustic Emissions:
To collect the best results, the infants were deep asleep on the lap of the caregiver inside a sound proof booth. The emissions were recorded initially on the left and then on the right ear. We checked technical conditions of the equipment, stability of the probe placed on the external auditory canal, characteristics of the stimulus to be presented, using “Quickscreen” and absence of environmental sounds during the test.
Caregivers were instructed to observe the auditory behavior of the infants and in cases in which we observed absence of transient otoacoustic emissions, they were instructed to come back for follow-up.
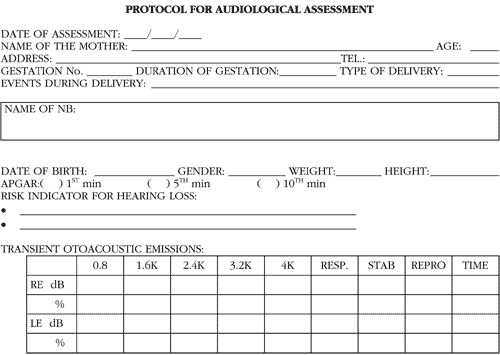
The collected results were recorded in a protocol (Annex 1) for posterior analysis and comparison.
The statistical analysis was conducted with t Student test for comparison of influence of the variable (with and without risk) on the studied parameters. We accepted significance level of 5% (or 0.05) for rejection of null hypothesis.Anex 1. Protocol for audiological assessment
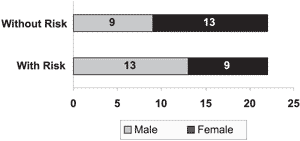
Graph 1. Infants according to gender
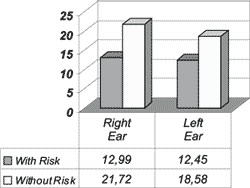
Graph 2. Mean general amplitude of response in dB for the Right Ear and the Left Ear in the group of infants with and without hearing loss risk indicators.
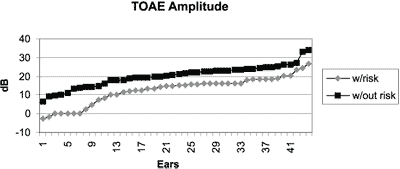
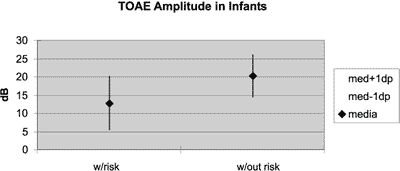
Graph 3. Distribution of amplitude of response in dB of transient otoacoustic emissions in infants with and without hearing risk.
Graph 4. Comparison between amplitude of responses of transient otoacoustic emissions in infant groups with and without hearing loss risk indicator.
RESULTS
We detected the occurrence of transient otoacoustic emissions by tested ear and defined comparison of general amplitude of response, reproducibility of waves A and B, probe stability and amplitude of responses for frequencies 0.8Hz, 1.6KHz, 2.4KHz, 3.2KHz and 4.0KHz, in addition to the existence or not of expressive differences between the tested ears (right and left).
Sample Characterization
Graph 1 shows the distribution of infants according to gender in the groups with and without hearing risk indicators and we observed in the group with risk, 9 female neonates and 13 male infants and in the group without risk, there were 13 female infants and 9 male infants.
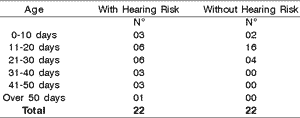
As to age, we observed that both groups were predominantly aged 11 to 30 days of life when performing the test, but the group with risk indicator for hearing loss had many infants tested when they were older than 30 days of life (Table 1).
Results obtained in Transient Otoacoustic Emissions
Graph 2 shows the mean general response amplitude for the right ear compared to the left ear (separately) in the groups of infants with and without risk indicator for hearing loss; it was possible to notice that in the group with risk indicator the amplitude was greater on the right (12.99 dB) than on the left (12.45 dB). The same predominance was observed in the group without risk indicator, being 21.72 dB for the right and 18.58 dB for the left.
Graph 3 shows the distribution of general amplitude in dB comparing the groups of infants with and without risk indicators for hearing loss and we observed a clear difference between the responses of those two groups, with tendency to lower intensity amplitudes in the group with risk indicator.
Graph 4 shows the mean in dB plus or minus one standard deviation of general amplitude of response in the groups of infants with and without risk indicator, being that we detected a statistically significant difference (p<0.01) between the groups. The group with risk indicator presented responses below those of the group without indicator, that is to say, the mean amplitude of response in the group without indicator was superior to the mean + 1 standard deviation of the group with risk indicator.
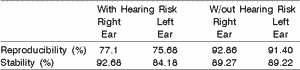
Upon comparing waves A and B reproducibility and probe stability in the external auditory canal by ear and between the two studied groups, it was possible to observe, as shown in Table 2, that the group with risk indicator had right ear reproducibility of 77.1% and on the left it was 75.68%, and stability was 92.68% on the right and 84.18% on the left. In the second group, without risk indicator, reproducibility on the right was 92.86% and on the left, 91.40% and stability was 89.27% on the right and 89.22% on the left.
The amplitude of response by frequency was tested with transient otoacoustic emissions between the right and left ears of the group with risk indicators for hearing loss as shown in Table 3, enabling the observation of greater amplitude in frequencies higher than 1.6kHz with peaks at 2.4 and 3.2 kHz.
In Table 4 we can see amplitude of responses by frequency tested with transient otoacoustic emissions between the right and left ears of the group without risk indicator and we observed a statistically significant difference between right and left ears (p<0.05) in frequency of 3.2kHz.
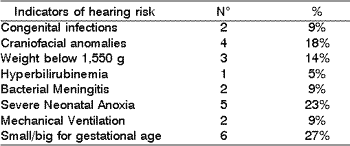
Table 5 shows the hearing risk indicators of the infants: congenital infections (9%), craniofacial anomalies (18%), weight below 1,500g (14%), hyperbilirubinemia (5%), bacterial meningitis (9%), severe neonatal anoxia (23%), mechanical ventilation (9%), small or big baby for the gestational age (27%).
We emphasize that in the group with hearing risk indicator there was absence of responses in 7 of the 46 tested ears. In the group without risk indicator, no infant failed the test.Table 1. Infants according to age.
Table 2. Comparison of the mean parameters: reproducibility and stability by ear and group with and without risk indicator.
Table 3. Amplitude by frequency of transient otoacoustic emissions between the Right Ear and the Left Ear in the group with risk indicator.
Table 4. Amplitude by frequency of transient otoacoustic emissions between the Right Ear and the Left Ear in the group without risk indicator.
Table 5. Infants risk indicators.
DISCUSSION
Graph 1 and Table 1 show that there was no gender predominance in any of the infant groups, but considering post-conceptual age we noticed that in the group with risk indicator, the test was conducted in some infants after 30 days of life, a fact that is justified by prolonged length of stay owing to birth conditions. Despite that, it was possible to follow the instructions given by Basseto (1998) of conducting hearing screening up to 3 months of life in all babies, allowing for early detection and appropriate follow-up of the cases.
The results of the general mean amplitude of responses for each ear in the groups with and without hearing risk can be observed in Graph 2. These responses showed smaller amplitude in the group with risk indicator compared to the group without risk, a fact that is in accordance with the findings by Vallejo (1999). Specifically speaking, in the group without risk, there was general amplitude of responses on the right and left ears of 21.78 and 18.58, respectively, values approximated to those found by Paludetti (1999) and Vallejo (1999).
In the group of infants with hearing risk, we found greater general amplitude on the right than on the left ear and the results were similar to those collected by Aidan et al. (1997).
The results described in Graphs 3 and 4 show greater amplitude of responses in the group without risk indicator when compared to the other group, both for description of responses and mean plus one standard deviation. Studies conducted by Kok et al. (1992) showed similar amplitudes in the group with no risk. Smurzyinski et al. (1993) found differences between the groups of newborns, being that lower responses were obtained in the group with risk for hearing loss.
Studies conducted by Aidan et al. (1995) and Newmark et al. (1997) indicated amplitude of responses greater on the right than on the left. This finding was also confirmed in our study in the frequency of 3.2kHz in the group without risk indicator. Previc (1991) in his study tried to justify the predominance of the right ear based on pre-natal development.
Table 2 shows greater reproducibility of the tests conducted in the group without risk indicator than in the other, that is, the general amplitude values of the group without risk indicator are greater, as well as the values for waves A and B reproducibility, differently from the group with risk. As to probe stability., this result was not repeated, since in both groups the stability percentage exceeded 70%, leading us to the conclusion that the tests were conducted under favorable conditions. Similar results were found in the study by Soares (1998).
In Tables 3 and 4 we observed that specific amplitude by frequency had a peak of amplitude at the region of 2.4 and 3.2kHz, and the main frequency of responses was in frequencies above 1.6kHz.
Risk indicators for hearing loss described in Table 5 show a great percentage of neonatal anoxia and small and large for the gestational age babies, similar findings to those reported by Silva et al. (1995). These results showed that a great portion of the indicators occurs in the perinatal period, justifying the importance of conducting neonatal hearing screening.
We emphasize that infants with cleft lip and palate had absence of otoacoustic emissions bilaterally, results that confirmed the reports by Lopes Filho & Carlos (1996) and Probst et al. (1991), who described the participation of middle ear in the production of otoacoustic emissions.
CONCLUSIONS
Through the analysis of results obtained during the conduction of this study, we concluded that infants with hearing risk indicator present a poorer performance in transient otoacoustic emissions than infants from the group with no risk, concerning parameters of waves A and B reproducibility, general amplitude and frequency-specific amplitude in the tested ear.
The transient otoacoustic emission test presents significant importance in the diagnosis of neonatal hearing loss, making us believe that this test should be part of the auditory assessment program of high risk newborns, together with auditory brainstem evoked potential, behavioral audiometry and immittanciometry.
The follow-up of hearing development of neonates with risk indicators for hearing loss should be considered despite the presence of otoacoustic emissions in the first test, since this test assesses specifically the cochlear function.
REFERENCES
1. Aidan D, Lestang P, Avan P, Bonfils P. Characteristics of transient-evoked otoacoustic emissions (TEOES) in neonates. Acta Otolaryngol 1997;117(1):25-30.
2. Aidan D, Lucek A, El-Bez M, Lestang P, Parrat S, Pascu A, Avan P, Bonfils P. Définition des critères de normalité d’une oto-émissio acoustique provoque chez le nouveau-né. Ann Otolaryngol Chir Cervicofac 1995;112:303-8.
3. Costa SMB, Costa Filho OA. Estudo das Emissões Otoacústicas Evocadas em recém-nascidos pré-termo. Rev Pró-fono 1998;1(10):21-25.
4. Denzin P. O efeito do ruído contralateral sobre a amplitude das emissões otoacústicas de produto de distorção em idosos. Rev Soc Bras ORL 1999;65(2):155-64.
5. Durante AS, Carvallo RMM. Emissão otoacústica transitória não – linear com estímulo contralateral em lactentes. Revista de Atualização Científica da Pró-Fono 2001;13(2), 271-276.
6. Eckley CA. Análise crítica das emissões otoacústicas produto de distorção em seres humanos. Dissertação de Mestrado. Faculdade de Ciências Médicas da Santa Casa de São Paulo. São Paulo, 1996.
7. Hood LJ, Hurlry A, Wen H, Berlin CI & Jackson DF. A new view of contralateral suppression of transient evoked otoacoustic emissions. Abstracts of the Sixteenth Midwinter research Meeting, Association for Research in otolaryngology 1993;16:102.
8. Joint Committee on Infant Hearing – Position statement. Audiology Today 1994;6(6):6-9.
9. Kemp DT, Bray P, Alexander L, Brown AM. Acoustic Emission Cochleography: Practical aspects. Scand Audiol Suppl 1986;25:71-95.
10. Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am 1978;64(5):1386-91.
11. Kok MR, Van Zanten Gª, Brocaar MP. Growth of evoked otoacoustic emissions during the first days postpartum. Audiology 1992;31:140-9.
12. Lopes Filho OC, Carlos R. Produtos de Distorção das emissões otoacústicas. Rev Bras de Medicina 1996;3(5):224-36.
13. Matas CG, Frazza MM, Munhoz MSL. Aplicação do Potencial Auditivo de Tronco Encefálico em Audiologia Pediátrica. In: Bassetto MCA et al. Neonatologia: um convite à atuação fonoaudiológica. São Paulo; 1998.
14. Mehl AL, Thomson V. Newborn hearing screening: the great omission. Pediatric 1998;101(1):E4.
15. Morlet T, Ferber-Viart C, Putet G, Sevin F, Duclaux R. Auditory screening in high-risk pre-term and full-term neonates using transient evoked otoacoustic emissions and brainstem auditory evoked potentials. Int J Pediatr Otorhinolaryngol 1998;45(1):31-40.
16. Newmark M, Merlob P, Bresloff I, Olsha M, Attias J. Click evoked otoacoustic emissions: inter-aural and gender differences in newborns. J Basic Clin Physiol Pharmacol 1997;8:133-9.
17. Oliveira JAA. O mecanismo eletrobiomecânico ativo da cóclea. Rev Bras de Otorrinolaringologia 1993;59(4):236-48.
18. Paludetti G, Ottaviani F, Fetoni AR, Zuppa AA, Tortorolo G. Transient evoked otoacoustic emissions (TEOAEs) in new-born: normative data. Int J Pediatr Otorhinolaryngol 1999;47(3):235-41.
19. Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol Ver 1991;98:299-334.
20. Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am 1991;89(5):2027-67.
21. Rhee CK, Park HM, Jang YJ. Audiologic evaluation of neonates with severe hyperbilirubinemia using transiently evoked otoacoustic emissions and auditory brainstem responses. Laryngoscope 1999;109(2):2005-8.
22. Silva AA, Maudonnet O, Panhoca R. A deficiência auditiva na infância. Retrospectiva de dez anos. ACTA AWHO 1995;14(2):72-5.
23. Vallejo JC, Oliveira JAA, Silva MN, Gonçalves AS, Andrade MH. Análise das Emissões otoacústicas transientes em crianças com e sem risco auditivo. Rev Bras de Otorrinolaringologia 1999;65(4).
24. Smurzynski J, Jung MD, Lefrenière D, Kim Dº, Kamath M, Rowe JC, Holman MC Leonard G. Distortion-product and click-evoked otoacoustic emissions of preterm and full-term infants. Ear haer 1993;14:258-74.
1 Fonoaudióloga Especialista em Audiologia Clínica pela Universidade de São Paulo.
2 Professora Doutora do Curso de Fonoaudiologia da Faculdade de Medicina da Universidade de São Paulo.
Centro de Docência e Pesquisa da Universidade de São Paulo.
Endereço para correspondência: Rua Borda do Campo, 274 Jardim do Mar
São Bernardo do Campo SP 09750-230
Tel (0xx11) 4330-7609 – Fax (0xx11) 4122-3227 – E-mail: pdenzin@bol.com.br
Article submitted on August 16, 2002. Article accepted on October 17, 2002