Year: 2002 Vol. 68 Ed. 6 - (11º)
Artigos Originais
Pages: 839 to 849
Tinnitus as a tool to study the central connections and the plasticity of the auditory system
Author(s):
Tanit G. Sanchez 1,
Maria C. Lorenzi 2,
Ana L. Brandão 3,
Ricardo F. Bento 4
Keywords: tinnitus, neural plasticity, somatic modulation, auditory pathways
Abstract:
The plasticity of the central nervous system allows functional rearrangement of the information carried between two or more centers, reinforcing or attenuating this information. Studying the central auditory pathways is fundamental for elucidation of die determinant factors upon sound perception and its modulation. Recent studies brought to light some important connections of the central auditory pathways and the somatosensory system. In this context, tinnitus represents an important tool in the evaluation of these connections once it can undergo modulation. Aim: To evaluate die influence of the somatosensory system upon the auditory system by studying tinnitus modulation (in patients) and genesis (in normal subjects) during muscle contractions, trying to establish risk factors to this phenomenon. Study design: Clinical prospective. Material and method: 121 consecutive patients with tinnitus and 100 healthy volunteers without tinnitus were studied. After the medical history and ENT exam, each subject underwent 16 different muscle contractions (head and neck and limbs) for 5 seconds each. In order to establish some risk factors, they were evaluated according to the audiometric pattern, well-defined etiologic diagnosis and symptoms of craniomandibular disorders. Results: There was a significant difference between die ability to modulate tinnitus (65,3%) and the ability to originate it in asymptomatic subjects (14,0%). Furthermore, die audiometric pattern, die presence of an etiologic diagnosis and the presence of craniomandibular disorders were not determinant factors for the ability of modulating tinnitus. Conclusions: Tinnitus modulation by the activation of the somatosensory system is overwhelmingly frequent. Further knowledge of die concerned mechanisms is necessary, once tinnitus modulation may be expression of the auditory and central neural plasticity.
![]()
INTRODUCTION
The concept of neural plasticity refers to the capacity the nervous system has to suffer functional abnormalities in its circuits that are more or less persistent1. To that end, it is necessary to assume that there may be morphofunctional modifications between the connections involving at least two nervous centers.
Throughout life, the exchange of information among the nervous centers goes through modifications, which may be enhanced or attenuated. It is not a matter of increasing or decreasing the number of cell elements (neurons) involved in each nervous pathways, but rather of reinforcing or attenuating the number and/or power of synaptic connections involved in information exchange between the two centers.
Various mechanisms of synaptic modulation have been involved in the expression of neural plasticity, among them sensitization and habituation. Both have been studied in sea slugs Aplysia californica, owing to the simplicity of its central nervous system2. Under normal conditions, tactile stimuli applied to the main structure of this small animal cause a reflex reaction of withdrawal of the gill into the shell. The phenomenon of habituation involves response learned suppression to repetitive stimuli, provided that the stimuli are considered innocuous, which causes a reduction in the power of previously existing connections and reduces the effectiveness of the response. In the example of the slug, repetitive and smooth touches (innocuous) resulted in gradual reduction of the withdrawal reflex, which can even disappear after some sessions, showing the response learned suppression. Conversely, sensitization involves learning a more effective response, determined by exposure to noxious stimuli that activate a group of inter-neuron facilitators. Thus, the same slug can present stronger and more effective withdrawal reflexes of the gill upon touch after the application of a small electrical shock to its tail. Using this simple example, we can notice that the synaptic activity in one or more neuronal circuits can be altered depending on the received stimuli, demonstrating the concept of neural plasticity.
Possibly the best example of auditory pathways plasticity is tinnitus. Considered as an excitatory neuronal activity that can be originated at any point of the auditory pathways (normally on the periphery), the tinnitus can suffer sensitization or habituation depending on the associations between the auditory pathways and other central nervous system centers. This mechanism has been well described in theory by the tinnitus neurophysiological model3, according to which the tinnitus is perceived as disturbance and distress only when the subject associated it with a noxious stimulus. This fact would be enough to promote activation of other nervous structures, such as the limbic system and the autonomic nervous system. In our interpretation, the correlations between nervous systems have probably always existed from an anatomical perspective, but they were only reinforced for the tinnitus information when it meant a noxious stimulus (sensitization). Clinical applicability of the neurophysiological model resulted in a treatment approach known as Tinnitus Retraining Therapy (TRT), which consists of the association of specific instructions and the exposure of the subject to low, neutral and continuous sounds (innocuous stimuli) for a prolonged period of time in order to reduce the response that the tinnitus causes in the auditory cortex (habituation)3.
The structures that form the auditory pathways have been anatomically well defined, even though not all anatomic-physiological correlations have been clearly explained yet. Many details about the central auditory pathways still have to be clarified, especially the factors that determine sound perception and modulation. Recently, some studies showed direct and indirect evidence of important connections of the central auditory pathways and other systems (visual, sensitive and somatosensory), and the tinnitus is almost always the instrument of these assessments, since it can be modulated by different stimuli4-6, as will be shown next. From a clinical perspective, one of these associations was initially established by Levine in 1999, when he described that 68% of the subjects with tinnitus could modulate it with quick head and neck muscle contractions7. As a result, clinical evidence of the connection of the central auditory and somatosensory pathways, activated by muscle contractions, was detected. The anatomical substrate for such a correlation had already been described by Wright; Ryugo in 1996, demonstrating that there was a great excitatory projection of nervous fibers from the gracilis and cuneiform nuclei (central somatosensory pathway) to the dorsal cochlear nucleus (central auditory pathway)8. Thus, during the path of the tinnitus towards the auditory cortex, the ascension of muscle information through the somatosensory system could cause a temporary influence in the perception of tinnitus, justifying its modulation.
Based on such data, the confirmation of the hypothesis that tinnitus can be modulated or originated by the stimulation of the somatosensory system (by means of muscle contractions) would contribute to partially explain the connections between auditory pathways and other central pathways. The scarcity of specific literature, the surprising frequency of tinnitus somatic modulation found by Levine, and its possible practical applications in the treatment, all influenced the desire to carry on the present study. The main objective of this study was to assess the influence of head and neck and limb muscle contractions by studying:
a. prevalence of subjects with tinnitus that can modulate it (experimental group);
b. prevalence of normal subjects that can originate it (control group);
c. proportion of abnormalities (modulation and generation) caused by the contraction of head and neck muscles compared to that caused by the contraction of limb muscles.
The secondary objective of this study was to define the possible risk factors for the modulation of tinnitus, comparing the patients’ data with and without normal pure tone audiometry, defined etiologic diagnosis and symptoms of mastication system dysfunction.
MATERIAL AND METHOD
We defined a case-control study by assessing patients with and without tinnitus between July 2000 and April 2001. This study was approved by the Ethics Research Committee of the Medical School, University of São Paulo (CAPPesq) in June 2000.
A. Experimental Group (Ge)
We initially created a group that consisted of 121 patients consecutively seen by the Group of Tinnitus of the Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of Sao Paulo. Out of the total, 77 patients (63.6%) were female and 44 (36.4%) were male patients. Ages ranged from 11 to 80 years, mean age of 51.78 and median of 52 years.
The inclusion criteria in Ge were: patients with constant unilateral or bilateral tinnitus, any gender and age, aware of the research study and having signed the informed consent term form. We excluded patients that were prevented from understanding the instruction by various reasons (mental retardation or other neurological diseases, psychiatric disorders, bilateral profound deafness not rehabilitated by oral methodology, etc.), conducting voluntary contraction movements (trismus, neurological or muscle diseases, etc.) or informing about the effects of the said movements on generating or modulating tinnitus (aphasia, muteness, etc.).
Each patient in the experimental group was submitted to a routine assessment defined by our unit (detailed history and complete ENT and audiological exams), followed by 16 movements of voluntary contraction of head and neck and limb muscles, maintained for 5 seconds each, as described by Levine 7. Such movements were conducted in a silent, but not soundproof, environment:
I. forced occlusion of mandible
II. counter-force against occipital pressure with the head at neutral position
III. counter-force against forehead pressure with the head at neutral position
IV. counter-force against the pressure on the vertex with the head at neutral position
V. counter-force against pressure on the mandible (closed mouth)
VI. counter-force against right temporal pressure
VII. counter-force against left temporal pressure
VIII. head rotation to the right with opposition of the right zygoma
IX. rotation of the head to the left with opposition of left zygoma
X. crossing the fingers of both hands, forcing them away from the body
XI. abduction of right shoulder against resistance
XII abduction of left shoulder against resistance
XIII. flexion of right thigh
XIV. flexion of left thigh
XV. abduction of the thighs
XVI. adduction of the thighs
To define the possible risk factors for tinnitus modulation, patients in the experimental group were divided into 3 pairs of subgroups, comparing the respective percentages of occurrence of these phenomena:
1. subgroup of patients with and without normal pure tone audiometry
2. subgroup of patients with and without defined etiologic diagnosis
3. subgroup of patients with and without symptoms of mastication system dysfunction
To identify the etiology of tinnitus we conducted the regular tests in our service: 1. pure tone audiometry and impedanciometry; 2. lab tests: complete blood count, fast glucose test, total cholesterol and fractions, triglycerides, T3, T4 and TSH, serologic reaction to syphilis. Occasionally, and according to the suspicion, we ordered autoimmune tests, glucose curve and 3-hour insulin reaction or serology to Lyme disease; 3. radiology: temporal bone computed tomography or inner ear and head magnetic resonance imaging.
To assess symptoms of mastication system dysfunction, we analyzed: 1. history of bruxism, headache, clicking when opening the mouth, and presence of oral habits (to put objects in the mouth, to chew gum, to bite the nails, etc.); 2. data from physical examination: sensitivity upon palpation of mastication muscles and clicking of temporal mandibular joint (TMJ) during opening and closing of the mouth.
B. Control Group (Gc)
Next, we proceeded to forming the control group, comprising 100 volunteers without tinnitus, with normal hearing or mild sensorineural hearing loss, matched by sex and age with the study group, equally aware of the study by the informed consent form. The exclusion criteria were the same adopted for the experimental group.
Each subject was submitted to the same 16 movements of muscle contraction of the experimental group and to the assessment of mastication system dysfunction, but no further tests were ordered.
Out of 100 patients in the control group, 57 (57%) were female and 43 (43%) were male subjects. Ages ranged from 19 to 80 years, mean age of 48.27 years and median of 46 years.
Data were analyzed as follows:
1. Percentage of subjects in the Ge that modulated tinnitus during one or more muscle contraction movements, assessing the frequency of tinnitus increase or decrease, or quality affection;
2. Percentage of subjects in the Gc that could temporarily originate tinnitus during one or more muscle contraction movements;
3. Comparison of prevalence of tinnitus modulation and generation between Ge and Gc, through t tests for proportions;
4. Proportion of tinnitus modulation (Ge) and generation (Gc) caused by contraction of the head and neck muscles compared to those caused by limb muscle movements, using 95% confidence intervals;
5. Relative risk (RR) of tinnitus development in normal subjects capable of originating tinnitus with muscle contraction, using odds ratio;
6. Correlation between tinnitus modulation and presence or absence of pure tone normal audiometry using chi-square test;
7. Correlation between tinnitus modulation and presence or absence of defined etiologic diagnosis, using the chi-square test.
8. Correlation between tinnitus modulation and presence or absence of mastication system dysfunction using chi-square test.
The level of significance was a < 0.05, as advocated for biological studies.
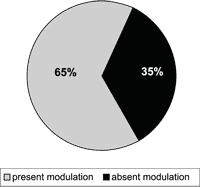
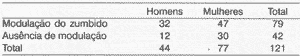
Figure 1. Modulation of tinnitus after muscle contractions in Ge (n = 121).
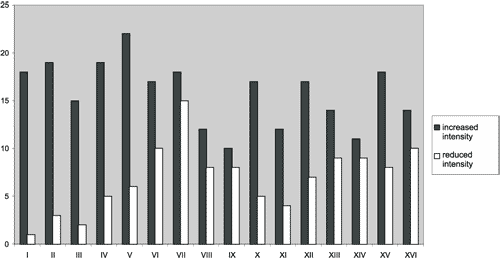
Figure 2. Distribution of tinnitus somatic modulation by movement.
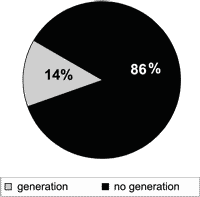
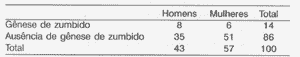
Figure 3. Generation of tinnitus during muscle contractions in Gc (n = 100).
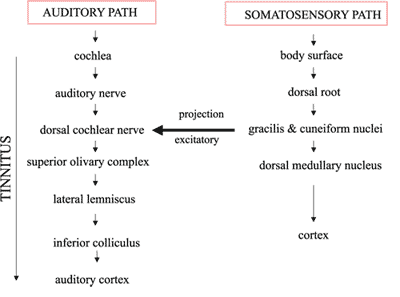
Figure 4. Diagram showing the influence of somatic pathways in auditory pathways through the dorsal cochlear nerve.
RESULTS
1. Percentage of subjects in the study group that modulated tinnitus during one or more voluntary muscle contraction movements, assessing frequency of tinnitus increase or decrease or quality modifications.
Analysis by patient:
Out of 121 subjects in the study group, 79 (65.3%) referred tinnitus modulation with the voluntary muscle contraction movements and 42 (34.7%) reported unaffected tinnitus (Figure 1). Out of 79 subjects with some modulation, 43 (54.4%) always presented increase in tinnitus, 11 (13.9%) always had decrease, 21 (26.6%) had both increase and decrease in the different movements and the remaining 4 patients (5.1%) modulated only tinnitus quality and not intensity.
Among the patients that presented symptom modulation, 16 (20.3%) had it in only one movement, 19 (24.1%) had it in two, and 44 (55.7%) had it in three or more movements.
Out of 121 patients, 55 (45.5%) presented bilateral tinnitus and out of these, 39 (70.9%) modulated tinnitus. Similarly, 66 patients (54.5%) presented unilateral tinnitus, being that 40 (60.6%) modulated it. Thus, there was no statistically significant difference in the capacity to modulate tinnitus among patients with unilateral or bilateral tinnitus.
Analysis by gender can be seen in Table 1. Considering only the male population, 72.7% of the patients presented modulation of tinnitus by movement of various muscle contractions; among women, this phenomenon was present in 61.0% of the cases. There was no statistically significant difference in tinnitus modulation among men and women (0.10 < p < 0.20).
Analysis by movement:
The greatest frequency of tinnitus modulation occurred in movements V (counter-force against pressure on the mandible - closed mouth) and VII (counter-force against left temporal pressure), followed in decreasing order by: VI (counter-force against right temporal pressure), II (counter-force against occipital pressure with the head at neutral position); IV (counter-force against the pressure on the vertex with the head at neutral position), XIII (flexion of right thigh); XV (abduction of the thighs); VIII (head rotation to the right with opposition of the right zygoma); X (crossing the fingers of both hands, forcing them away from the body); XVI (adduction of the thighs); IX (rotation of the head to the left with opposition of left zygoma); XII (abduction of left shoulder against resistance); XIV (flexion of left thigh); I (forced occlusion of mandible); III (counter-force against the forehead pressure with the head at neutral position); XI (abduction of right shoulder against resistance).
No movement was always uniform in its effects on tinnitus in the various patients; all of them (100%) caused increased in symptoms in some patients and decrease in others (Figure 2).
2. Percentage of subjects in the control group that could originate tinnitus temporarily during one or more movements of voluntary muscle contraction.
Analysis by patient:
Out of 100 subjects in the control group, only 14 (14.0%) originated tinnitus perception with the voluntary muscle contraction movements (Figure 3).
The analysis by gender showed that this phenomenon occurred in 8.0% of the male subjects and in 6.0% of the female subjects (Table II). There was no statistically significant difference detected here (0.20 < p < 0.30).
Analysis by movement:
The greatest frequency of tinnitus generation occurred with movements I (forced occlusion), VIII (head rotation to the right with opposition of the right zygoma), and IX (rotation of the head to the left with opposition of left zygoma). Next, in decreasing order of frequency, we detected the following movements: IV (counter-force against the pressure on the vertex with the head at neutral position), V (counter-force against pressure on the mandible closed mouth), VI (counter-force against right temporal pressure), VII (counter-force against left temporal pressure), XV (abduction of the thighs), III (counter-force against the forehead pressure with the head at neutral position), X (crossing the fingers of both hands, forcing them away from the body), XVI (adduction of the thighs), II (counter-force against occipital pressure with the head at neutral position), XII (abduction of left shoulder against resistance), XIII (flexion of right thigh), XIV (flexion of left thigh), XI (abduction of right shoulder against resistance).
3. Comparison of the prevalence of tinnitus modulation or generation between Ge and Gc.
There was statistically significant difference (p<0.01) between the capacity to modulate tinnitus of the experimental group (65.3%) and the capacity to originate tinnitus by the asymptomatic subjects of the control group (14.0%).
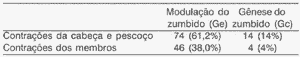
4. Proportion of modulation (Ge) and generation (Gc) of tinnitus caused by contraction of head and neck muscles compared to those caused by limb muscle contraction.
In the experimental group, tinnitus modulation after head and neck contraction movements was detected in 74 patients (61.2%; CI (95) = 52.6 to 69.8%) and after limb muscle contraction in 46 subjects (38.0%; CI (95) = 29.4 to 46.6%). The analysis of 95% confidence interval showed statistically significant difference among the results obtained for both types of muscle contraction. In the control group, the results were different: out of 14 volunteers (14.0%; CI(05) = 7.1 to 20.9%) that caused tinnitus generation, all of them were caused by head and neck muscles, whereas only 4 of them (4.0%; CI (95) = 0 to 8.0%) repeated it with limb muscle contractions (Table III). The analysis of 95% confidence intervals also showed statistically significant difference among the results obtained for both types of muscle contractions.
5. Relative risk (RR) of developing tinnitus in normal subjects capable of originating tinnitus by muscle contraction.
Data of exposure to the factor “capacity of generating tinnitus from voluntary muscle contractions” observed the risk that this capacity represents to the development of the tinnitus symptom, and it can be observed in Table IV.
The odds ratio for tinnitus modulation and/or generation in this case was calculated as 11.19, meaning that a patient that has tinnitus presents 11 times greater likelihood of modulating/generating tinnitus by muscle contractions than a normal subject. Thus, it is estimated that normal subjects capable of generating tinnitus by voluntary muscle contractions have a very high relative risk of developing tinnitus.
6. Relation between modulation of tinnitus and presence or absence of normal pure tone audiometry.
The analysis of pure tone audiometry showed abnormal results in 116 of the 121 patients of Ge (95.9%), and normal results in only 5 cases (4.1%). Considering only the 116 people who had abnormal audiometry, there was tinnitus modulation in 76 subjects (65.5%). Similarly, among the 5 normal hearing subjects, 3 (60%) had tinnitus modulation.
The presence of audiometric abnormalities did not prove to be a determining factor in the capacity of modulating tinnitus with the proposed muscle contractions, since Fisher’s exact test did not show statistically significant difference in the distribution of modulation capacity according to the observed audiometric patterns (p= 0.34).
7. Relation between tinnitus modulation and presence or absence of defined etiologic diagnosis.
The analysis of etiologic diagnosis showed defined etiology in 84 of the 121 patients in Ge (69.4%), being that the remaining 37 (30.6%) were considered as idiopathic tinnitus. Considering only the 84 subjects with defined etiology, there was tinnitus modulation in 52 subjects (61.9%). Similarly, among the 37 subjects with idiopathic tinnitus, 27 (73.0%) presented tinnitus modulation.
Definition or not of tinnitus etiology was not a determining factor in the capacity of modulating tinnitus through muscle contractions, since the chi-square test did not show statistically significant difference in the distribution of the capacity to modulate according to the presence or absence of defined etiologic diagnosis (0.20 < p < 0.30).
8. Relation between tinnitus modulation and presence or absence of mastication system dysfunction.
Symptoms of mastication system dysfunction were present in 68 of the 121 patients of the Ge (56.2%), out of which 46 modulated tinnitus (67.6%). Among the 53 without symptoms (43.8%), 33 (62.3%) presented tinnitus modulation. There was no statistically significant difference among patients with and without symptoms of mastication system dysfunction considering the capacity to modulate tinnitus (p > 0.50).Table I. Table of contingency for the study of tinnitus modulation in both genders in Ge.
Table II. Table of contingency for the study of tinnitus genesis in both genders in Gc.
Table III. Assessment of effectiveness of head and neck and limb muscle group contraction in tinnitus modulation and generation.
Table IV. Data concerning odds ratio for the development of tinnitus as a symptom, considering the capacity of generating tinnitus from voluntary muscle contractions.
DISCUSSION
Tinnitus affects about 15% of the population9. It may be caused by various reasons, be them metabolic, otological, neurological, cardiovascular, pharmacological, dental and psychological, which can be present concomitantly in the same subject10-15.
The presence of tinnitus frequently becomes a factor of great negative repercussion in the life of the subject: sleeping gets difficult, concentration in daily and professional activities reduces, social life is compromised, in addition to affecting significantly the emotional balance, leading to states of anxiety and depression10-15. Pathophysiology is very complex and has not been fully understood yet, which compromises partially the evolution of treatment. Therefore, contributions to better understand tinnitus are extremely useful since they enable the development of treatment and better comprehension of hearing pathways and important central connections.
A. Relations between the hearing systems and other systems.
One of the few consensus in the literature about tinnitus is that it is the result of aberrant neuronal activity in the auditory pathways, normally of excitatory nature3, 16, 17, which is mistakenly interpreted as a sound at the auditory cortex. Thus, the study of the generation, perception and modulation of tinnitus demonstrates better the auditory pathway itself, as well as the interaction with other non-auditory systems.
A sequence of recent studies contributed a lot to reinforce the existence of other systems that interfere in the modulation of tinnitus. After concomitant total unilateral deafferentation of the auditory and vestibular pathways 3 distinct forms of tinnitus were demonstrated, which can be originated or modulated by means of sensitive afferent stimulus of the skin hand (cutaneous evoked tinnitus - CET), by vision (gaze-evoked tinnitus – GET) or by finger movement (finger-evoked tinnitus – FET).
Cacace et al. (1999) reported 2 cases of unilateral total ablation of auditory and vestibular pathways during neurosurgery for skull base and posterior fossa tumor. They referred generation of tinnitus evoked by cutaneous stimulation of the upper portion of the hand and fingers ipsilaterally to the deafferentation. Thus, they demonstrated strong evidence that there are connections between central auditory pathways and sensitive systems through the cutaneous stimulation of hand and fingers18.
Lockwood et al. (2001) stated that tinnitus gaze-evoked tinnitus (GET) can occur about one year after neural deafferentation in surgeries for cerebellum-pontine angle tumors, and there were 17 cases described up to 2001. Considering mapping of the central sites involved in GET, the authors analyzed the PET scans of 8 subjects with GET and 7 normal volunteers matched by age and sex. They identified that the activated areas in these subjects were the lateral regions of the pons, or the auditory cortex. In the side ipsilateral to deafness (intraoperative neural ablation), they noticed expansion of the cortical areas responsive to pure tones presented to the normal ear, confirming one more example of central nervous system plasticity. Thus, they concluded that the patients with GET after total unilateral deafferentation developed plastic abnormalities that enabled stimulation of the auditory pathway by the neural activity associated with eye movements6.
In 2001, Cullington described the case of a 78 year-old patient with severe sensorineural loss and bilateral tinnitus for 30 years. However, for 2 years the patient had noticed an additional whistle in the left year that was clearly associated with the movement of the middle finger of the ipsilateral hand: it was only present when he moved the finger upward and downwards, there were no signs of fatigue and the quicker the movement, the louder was the tinnitus intensity4. Even though this patient had not undergone neural ablation, the prolonged profound deafness could have been the factor that stimulated neural plasticity of the central nervous system up to the extent of forging connections for the regeneration of other systems. What is not known is whether this would be an appropriate connection, with unknown role or similar to synkinesis that can occur during the regeneration of facial nerve. Regardless, they seem to mirror the connections of the central auditory pathways that may have been previously in existence from an anatomical perspective, but had been recruited only for functional applications in the recent period.
B. Relation between the auditory system and the somatosensory system justifying the phenomenon of tinnitus modulation.
Auditory pathways are formed by well structurally determined centers, even though not all of the anatomical and physiological correlations among them have been completely understood. The cochlear nucleus is the first central nucleus of the auditory pathways and it receives information from the cochlear sensorial cells through the auditory nerve. In the highest point of the auditory pathway, the lemniscal system sends the received information to the primary hearing areas. At the same time, the extra-lemniscal portion of the ascending pathway also transmits auditory information that projects to the areas of association19. Many neurons of the extra-lemniscal system receive information from other sensorial tracts such as for example the somatosensory system20 showing a relation between the two systems 21, 22.
Cuneiform and gracilis nuclei form together the dorsal medullary nucleus that occupies a position in the somatosensory system that is analogous to the position of the cochlear nucleus in the auditory system, receiving information directly from the dorsal root, which in turn had received information from the proprioceptive, tactile and vibration receptors of the body surface8. The lateral cuneiform nucleus is where neck afferent fibers arrive from the ear and the suboccipital muscle that provides information about head and the pinna positions necessary to process acoustic information8.
Sporadically, some patients report spontaneously that the tinnitus they hear is influenced temporarily by muscle contractions, such as masseter and sternocleidomastoid contractions. Recent studies showed that when patients are directly asked about that, subjects with tinnitus reported that it was not that sporadic an event. Moller et al., in 1992, described that nearly 40% of the patients presented some modulation of tinnitus with stimulation of the ipsilateral median nerve (increase in 15% and reduction in 23% of the cases)19. According to Rubinstein (1993), approximately one third of the patients present tinnitus abnormalities as a result of movements or pressure on the TMJ 23. Finally, Levine (1999a) reported that 68% of the patients presented some type of modulation in tinnitus characteristics when submitted to different muscle contraction movements involving head and neck and limb muscles 7.
Based on the existence of mutual connections between the hearing system and the somatosensory system, Wright and Ryugo (1996) experimentally proved that there is a great projection of the cuneiform nucleus over the cochlear nucleus, which favors the hypothesis of excitatory stimulation over the cochlear nucleus8.
Since muscle contractions are a form of activation of the somatosensory system, this anatomical link between the two systems could justify the influence of some voluntary muscle contractions present over some types of tinnitus. Based on these findings, Levine (1999a) submitted 70 patients with tinnitus consecutively seen to perform voluntary muscle contraction movements involving the head, neck and limbs, reaching a prevalence of 68% of subjects capable of modulating tinnitus during these contractions. In our study, we had similar results (65.3%), showing that more than half of the patients that modulated tinnitus referred increase in tinnitus intensity (54.4%), which could be justified by the anatomical findings by Wright and Ryugo. Despite these characteristics, which suggest the existence of excitatory neuronal activity in the somatosensory system over the auditory one, some electrophysiological studies in cats suggested that the final effect of cuneiform nucleus activation could be the inhibition of the dorsal cochlear nucleus24, 25, justifying the reduction of the tinnitus intensity detected in some patients.
Even though our results related to modulation of tinnitus had been similar to those by Levine, they are certainly greater than those by Moller et al.9 and Rubinstein23, which demonstrated the presence in, respectively, 40% and 30% of the cases. However, the stimulation of the somatosensory system was different in each study, since Moller et al. used the electrical stimulation of the median nerve and Rubinstein used movements or pressure of the TMJ. Thus, this difference should be carefully interpreted.
The definite explanation for this modulation phenomenon is still controversial. Considering the tinnitus as the result of an aberrant neuronal activity of the auditory pathways 3, 16, 17, Levine suggested that somatic stimuli coming from head and neck muscle contractions could, through the polysynaptic path, uninhibited the ipsilateral dorsal cochlear nucleus7, generating excitatory neuronal activity in the auditory pathways that leads to the tinnitus (Figure 4).
The comparison of these findings between the genders did not show any statistically significant difference concerning prevalence of modulation and genesis of tinnitus during movements, which was expected from a clinical perspective.
In our study we included a control group to study the possibility of originating tinnitus in normal subjects, considering the phenomenon of tinnitus genesis in these subjects as the same expression of the tinnitus modulation phenomenon in symptomatic subjects. The head and neck movements produced greater effect in tinnitus modulation and generation than limb movements in both groups, in agreement with the findings by Levine7, even though this author did not have a control group. Both the genesis and modulation of tinnitus are probably related to a greater anatomical and physiological interaction between the somatic pathways of these regions and auditory pathways at the level of the dorsal cochlear nucleus. The fact that most subjects (55.7%) modulate the tinnitus through 3 or more different muscle contraction movements reinforced the likelihood of having a wealth of connections between the two systems. Conversely, neuroanatomy can justify the low efficacy of limb muscle contraction in triggering the studied phenomenon, since the connections between the two systems at the level of the limbs can activate concomitantly other muscle groups of the head in some patients, facilitating the modulation/generation of tinnitus in these cases and making it more difficult in others.
Upon the assessment of the control group, we observed that 14% of the subjects who previously did not have tinnitus presented the capacity to temporarily generate it during muscle contraction movements, also preferably from head and neck muscles. The possibility of uninhibited cochlear nucleus or the occurrence of excitatory activity of the auditory pathways by activation of the somatic pathways of these regions can represent the base that supports this fact.
Thus, taking for granted that the capacity to originate tinnitus (in normal subjects) and to modulate it (in subjects with tinnitus) is caused by the same mechanism, the capacity to originate the tinnitus (expression of an activity of leading to uninhibited or somatic excitation of the auditory paths) could represent a risk factor for the future development of tinnitus, as of the moment the subjects start to be able to modulate it.
Conversely, the small percentage of tinnitus generation in this group, as well as the lack of tinnitus modulation in about one third of the subjects in the experimental group, could be justified by the inappropriate activation of the muscle groups responsible for interfering in the cochlear nucleus7 or the inability to notice subtle abnormalities in tinnitus sound caused by the fact that they were not in a sound treated environment.
C. Factors associated with the modulation phenomenon
Ass previously said, Levine’s study showed that 68% of the 70 patients presented some type of modulation in its characteristics, even though these findings had occurred regardless of tinnitus etiology or audiometric results of patients7. Movements were used in our study as described by the author and our data were similar concerning prevalence of tinnitus modulation (65.3%) and risk factors. Thus, we did not find either a correlation between tinnitus modulation and audiometric findings or etiologic diagnosis definition.
Even though hearing loss in a concomitant symptom in 85 to 96% of the patients with tinnitus5, 26-29, many questions are still unanswered: why do some patients with the same hearing loss develop tinnitus whereas others not? What determines the moment of tinnitus generation in progressive loss? Why do patients with bilateral symmetrical hearing loss can present unilateral tinnitus? By trying to answer these questions, Levine proposed that the tinnitus presents a somatic component7, since of all sensorial systems, the somatosensory is the only one possibly related to tinnitus, as can be seen in some patients with tinnitus and dysfunction of the mastication system by TMJ disorder or still, in cases of cervical lesion by whiplash. According to the author, some characteristics of tinnitus can make us suspect of a somatosensory component in its origin, such as for example: a) the association of a somatosensory head and neck lesion; b) the ipsilateral location of the tinnitus considering the somatic lesion; c) absence of vestibular symptoms and neurological test abnormalities; d) the presence of unilateral tinnitus with bilateral symmetrical pure tone audiometry, normally within the normal range7.
Considering the prevalence of modulation found in both studies conducted in a very careful fashion, we believe it is possible that a somatic component should be investigated, especially in patients with dysfunction of the somatosensory system ipsilateral to the tinnitus. Even though we agree that normal pure tone audiometry can reinforce this possibility, the presence of a hearing loss does not exclude this suspicion, since it is very possible that a subject has tinnitus triggered by somatic lesions and a hearing loss of other etiology.
Even though there is no direct correlation with the topic studied here, it was interesting to see, considering the etiological diagnosis, that only 29.7% of the patients in the experimental group presented idiopathic tinnitus, a fact that we attributed to our systematic search for likely etiologic diagnosis for all cases. However, it should be pointed out that according to our results, the definition of the etiologic diagnosis is irrelevant for the manifestations of modulation phenomenon. It would be important to find out what are the common predisposing factors to these subjects, what might be explained by anatomical and functional characteristics by the auditory pathway and its connections.
In our study, we investigated the possible correlation of the tinnitus modulation capacity and presence of TMJ dysfunction symptoms, which was not confirmed either. However, according to the literature, the spasms of mastication muscles30 or abnormal TMJ 7, 23 could originate or maximize a preexisting tinnitus. Rubinstein noticed that one third of the patients with cranial-mandible dysfunction had tinnitus modulation with mandibular movements or TMJ pressure23. Even though it does not justify our findings, the author reinforced the importance of having a correlation between tinnitus and muscle contraction interfering in the episode. There are cases in which the physician says to the patient that there is nothing to be done, the patient has to learn how to live with the tinnitus, but they may be contributing to the vicious cycle in which muscle tension aggravates tinnitus12, a fact to which we entirely agree.
The surprising high prevalence of the somatic modulation phenomenon makes us wonder whether it is a characteristic of the subjects with tinnitus. Further studies will determine if the patients could benefit from specific forms of treatment involving mainly head and neck muscles and using physical therapy, acupuncture, electrical stimulation, among others. Thus, we emphasize the importance of a broader investigation of the patient with tinnitus trying to detect suggestive signals of somatosensory modulation.
CONCLUSIONS
The deep study of tinnitus characteristics can be a good parameter to demonstrate the plasticity of the central nervous system and the connections of the auditory pathways and other systems. In the present study it was possible to demonstrate that there is clinical evidence of interaction between the auditory and somatosensory pathways, since the prevalence of subjects with tinnitus that can modulate it during voluntary muscle movements is surprisingly high (65.3%), with statistically significant difference concerning the prevalence of normal subjects that can originate tinnitus during the same movements (14%). In addition, the contraction movements involving head and neck were more effective to cause tinnitus modulation and generation than limb muscle movements, suggesting the presence of anatomical connections between the auditory system and the somatosensory one, more at the head level than at the limb level.
Conversely, normal pure tone audiometry, defined etiologic diagnosis and symptoms of mastication system dysfunction were not determining factors in the capacity to modulate tinnitus.
ACKNOWLEDGMENT
The authors would like to thank Gaby Cecília Yupanque Guerra, a physician living in Peru, for her significant participation in the previous stage of this work.
REFERENCES
1. Kandel ER. Nerve Cells and Behavior. In: Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science, 4th Ed. USA: Mc Graw-Hill; 2000.
2. Kupfermann I. Learning and Memory. In: Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science, 4th Ed. USA: Mc Graw-Hill; 2000.
3. Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neuroscience Research 1990;8:221-54.
4. Culington H. Tinnitus evoked by finger movement: brain plasticity after peripheral deafferentation. Neurology 2001;56(7):978-9.
5. Fowler EP. Head noises in normal and in normal and disordered ears: significance, measurement, differentiation and treatment. Arch Otolaryngol 1944;39:498.
6. Lockwood AH, Wack DS, Burkard RF, Coad ML, Reyes SA, Arnold SA, Salvi RJ. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology 2001;56:472-80.
7. Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol 1999b ;20(6):351-62.
8. Wright DD, Ryugo DK. Mossy Fiber Projections From the Cuneate Nucleus to the Cochlear Nucleus in the Rat. The Journal of Comparative Neurology 1996;365:159-72.
9. National Institutes of Health. National Strategic Research Plan: Hearing and Hearing Impairment. Bethesda: U.S. Department of Health and Human Services;1996.
10. Sanchez TG. Zumbido: estudo da relação entre limiar tonal e eletrofisiológico e das respostas elétricas do tronco cerebral. São Paulo, 1997 (Tese de Doutorado, Faculdade de Medicina da Universidade de São Paulo).
11. Sanchez TG, Balbani AP, Bittar RSM, Bento RF, Câmara J. Lidocaine test in patients with tinnitus: Rationale of accomplishment and relation to the treatment with carbamazepine. Auris Nasus Larynx, 1999a;26:411-17.
12. Sanchez TG, Bento RF, Miniti A, Câmara J. Zumbido: Características e Epidemiologia. Experiência do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. Rev Bras Otorrinolaringologia 1997a;63(3):229-235.
13. Sanchez TG, Bittar RSM, Ajimura F, Bento RF. The management of persistent tinnitus after the treatment of sudden deafness: the effect of intravenous lidocaine and oral carbamazepine. Proceedings of the Sixth International Tinnitus Seminar. Cambridge, UK: ed. Hazell, JP; 1999b. p.534-7.
14. Sanchez TG, Pedalini MEB, Bento RF. Hiperacusia: Artigo de revisão. Arq Fund Otorrinolaringol 1999c;3:184-88.
15. Sanchez TG, Zonato AY, Bittar RSM, Bento RF. Controvérsias sobre a Fisiopatologia do Zumbido. Arq Fund Otorrinolaringol 1997b;1(1):2-8.
16. Moller AR. Pathophysiology of tinnitus. Ann Otol Rhinol Laryngol 1984;93:39-44.
17. Moller AR, Moller MB, Jannetta PJ, Jho HD. Compound Action Potentials recorded from the exposed eighth nerve in patients with intractable tinnitus. Laryngoscope 1992a;102:187-197.
18. Cacace A, Cousins J, Parnes S, Semenoff D, Holmes T, McFarland DJ, Davenport Categbauer K, Lovely TJ. Cutaneous-evoked tinnitus: I. Phenomenology, psychophysics, and functional imaging. Audiol Neurootol 1999;4:247-57.
19. Moller AR, Moller MB, Yokota M. Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope 1992b;102:1165-71.
20. Aitkin L. The auditory midbrain, structure and function in the central auditory pathway. Clifton NJ: Humana press; 1986.
21. Hotta T, Kameda K. Interactions between somatic and visual or auditory responses in the thalamus of the cat. Exp Neurol 1963;8:1-13.
22. Thompson RF, Smith HE, Bliss D. Auditory, somatic, sensory, and visual response interactions and interrelations in association and primary cortical fields of the cat. J Neurophysiol 1963;26:365-378.
23. Rubinstein B. Tinnitus and craniomandibular disorders – is there a link? Swedish Dental J Supplement 1993, 95:1-46.
24. Young ED. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain Res 1980;200:23-37.
25. Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol 1995;73:743-765.
26. Antonelli A, Bellotto R, Grandori F. Audiologic diagnosis of central versus eighth nerve and cochlear auditory impairment. Audiology 1987;26:209-226.
27. Barnea G, Attias J, Gold S, Shahar A. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology 1990;29:36-45.
28. Reed GF. An audiometric study of two hundred cases of subjective tinnitus. Arch Otolaryngol 1960;71:74-84.
29. Shea JJ, Emmett JR. The medical treatment of tinnitus. J Laryngol Otol Suppl 1981;4:130-138.
30. House PR. Personality of tinnitus patient. In: D. Evered, G. Lawrenson (Eds) Tinnitus. Ciba Foundation Symposium London: Pitman 1981;85:193-198.
1 Collaborating Professor, Medical School, University of São Paulo, Assistant Physician and Ph.D.,
Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
2 Ph.D. in Medicine, Medical School, University of São Paulo, Researcher with the Division of Clinical
Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
3 Trainee, Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
4 Associated Professor, Discipline of Otorhinolaryngology, Medical School, University of São Paulo.
Study conducted at the Division of Clinical Otorhinolaryngology, Medical School, University of São Paulo.
Address correspondence to: Dra. Tanit Ganz Sanchez – Rua Pedroso Alvarenga, 1255 cj. 27
Itaim Bibi São Paulo SP 04531-012
Tel (55 11) 3167-6556 Fax (55 11) 3079-6769 – E-mail: tanitgs@attglobal.net
Artigo recebido em 04 de setembro de 2002. Artigo aceito em 17 de outubro de 2002.