Year: 2003 Vol. 69 Ed. 3 - (14º)
Artigo Original
Pages: 377 to 384
Phonoaudiological findings in patients submitted to hypoglossal-facial anastomosis
Author(s):
Elisabete C. C. F. Silva 1,
José R.G. Testa 2,
Yotaka Fukuda 3
Keywords: peripheral facial paralysis, facial hypoglosal anastomosis, chew, swallowing, speech, dysphage
Abstract:
The hypoglossal-facial anastomosis (HFA) have been related in patients with facial nerve lesion where proximal segment more other surgical produceres had been faited or had not been possible success. Aim: The objective of the present research is to verify the evidence of mobility in the phonoarticulate organs, speech function, chew and swallowing in patients sujected to HFA. Study design: Clinical prospective. Material and method: Eight patients with peripheral facial paralysis (PFP) were evaluated and subjected to FIFA at UNIFESP/EPM in the period from 1989 to 2000, with 6 females and 2 males, aged between 21 and 71 years with an average of 50 years. Of these, 5 after exeresis of Acoustic Neurinoma, 1 after exeresis of Fibrosarcoma, 1 after a gunshot wound and 1 after idiopathic peripheral facial paralysis of poor evolution. In the phonoaudiological evaluation, the protocol used involved identification data; classification of the facial nerve; treatments carried out; facial symmetry in repose and on voluntary movement; synhinesis of the eyes, mouth, nose and cheeks; phonoarticulate and tongue disorders; changes in chew and of the palate and a questionary concerning the appearence of the respective disturbances. Results: The degree of pos anastomosis and reabilitation ranged to the eyes between II and V and to the mouth between III and V (House & Brakemann, 1985). We carne to the conclusion that the recover was satisfactory and important but patients' recover expectation were inferior. There have been noted: articulatory imprecision chewing disfunction, deficit sphincteral function of oral muscles and disphage.
![]()
INTRODUCTION
Humans' face serves our "first impression". We can express our feelings through movement and facial mimics, showing happiness, sadness, crying, anger and laughing, associated or not with verbal communication.
The presence of facial paralysis can lead a person to psychological, social and even to professional problems. In addition, abnormalities such as lubrication of the affected eye, affections of speech articulation and swallowing are found to impair the functions. Peripheral facial paralysis (PFP) can arise from a trauma, infection, tumor, congenital or idiopathic origin.
In the presence of PFP, there are a number of prognostic tests that are indicated, such as electroneurography and Higer test, which evidence the motor action potential of the nervous fibers of the facial nerve 1, 2. Among many surgical treatments, the most indicated ones are facial nerve decompression, grafting and hypoglossal-facial anastomosis (HFA). Medication therapy is reserved for acute stage. Speech and voice therapy aims at rehabilitating facial mobility and integrating the patient in society.
HFA has been used as a surgical procedure for the treatment of facial paralysis with minimum or no recovery. One of the surgical indications of HFA is exeresis of vestibular nerve Schwannoma (8th nerve neurinoma)3, when there is unilateral facial paralysis leading to facial asymmetry, lack of facial muscle movement, associated with synkinesis and spasms 4, 5. Other indication would be the cases in which the proximal portion of the facial nerve is non-useable.
After HFA, the eye closing function has been mentioned by many authors, which commented on the competence of the orbicular ocular muscle for complete or almost complete voluntary closure 7. However, there is the need to lubricate the eye, up to the moment it allows complete closure 3.
As to speech and mastication, various studies have reported that after HFA the patient had movements of muscle mass associated with tongue movement in eating situations and for a short period of time 8.
Despite the presence of hemitongue atrophy favoring movement inability, swallowing function did not prove to be abnormal 4, 7 even for liquid and/or paste consistency 8, with the possibility of improving after 2 or 3 months 4, and affection of articulation of words.
All patients submitted to HFA presented grade VI facial paralysis. After surgery, patients were submitted to speech and voice assessment.
The purpose of the present study was to check mobility of speech structures, speech, mastication and swallowing and the expectation of improvement in patients submitted to hypoglossal-facial anastomosis.
MATERIAL AND METHOD
We assessed 8 patients with PFP submitted to HFA and followed up in the Outpatient Department of Otorhinolaryngology (Unit of Facial Nerve Paralysis) of Hospital São Paulo, Federal University of São Paulo, between 1989 and 2000. They spontaneously underwent speech and voice assessment and there were 6 female patients and two male patients. Among the studied cases, the age ranges were 21-30 years (2 patients); 31-30 years (1 patient); 41-50 patients (2 patients), 51-60 years (2 patients) and 71-80 years (1 patient).
Among them, 4 patients had PFP on the left and 4 on the right. HFA was indicated in 5 patients with PFP post-exeresis of vestibular nerve Schwannoma, one with PFP after fibrosarcoma exeresis, 1 with PFP after gunshot injury, and 1 with idiopathic PFP of poor evolution. All patients were periodically followed by the ENT physician and they detected improvement and grade of PFP, based on the classification by House & Brakemann (1985).
As to speech and voice assessment, we developed an assessment protocol comprising:
1) Identification data (name, gender, date of birth, age, hospital registration number, race, profession, address and telephone number), and history (date of onset and side of PFP, description of onset, signs and symptoms, stapedial reflex, lachrymation, surgery and past history);
2) classification of recovery of facial nerve and ENT management (decompression, gold weight on the eyelid, use of eye drops and/or eye cream);
3) treatments performed (medication use, physical therapy, speech and voice therapy);
4) postoperative speech and voice assessment, comprising
a) facial rest asymmetry (nasal-labial fold, eyes and mouth);
b) symmetry during voluntary movement of facial expression by elevating frontal muscle, closing eyes slowly, laughing with the opened mouth, lip protrusion and cheek inflation, total asymmetry, severe, moderate, mild and absent;
c) almost complete and complete movements;
d) facial synkinesis was classified as no synkinesis, little synkinesis, evident synkinesis without deformation and total synkinesis;
e) speech and articulation disorders, asking the patients to verbally utter numbers 1 to 10, days of the week and months of the year;
f) tongue mobility disorders related to elevation, depression, lateralization (right and left); rotation (clockwise and anti-clockwise), vibration;
g) taste, mastication and swallowing disorders;
h) speech and voice management;
i) Questionnaire: Do you think that facial movements have improved after the surgery? Yes/No; Do you think that the surgery favored your speech? Yes/No; Do you think the surgery favored mastication? Yes/No; Do you think that surgery favored swallowing? Yes/No.
All patients had an ENT visit and later they underwent speech and voice assessment, following the protocol referred above; they were sitting down and were asked to repeat and execute facial mimics. In addition, we obtained authorization to take picture during the assessment.
RESULTS
All assessed patients reported in the present study were grade VI (severe) PFP before HFA.
It is important to point out that patients numbered 6 and 8 in all tables were recently submitted to HFA with few detectable results.
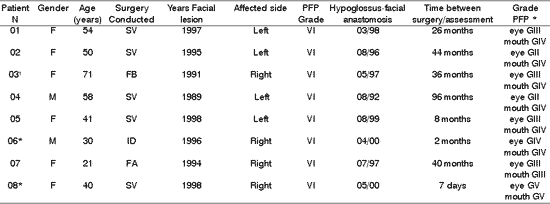
The results are demonstrated in Tables 1-10.Table 1. Distribution of 8 Patients with PFP relating to age, type of surgery, date, facial side affected by peripheral facial paralysis, grade of lesion after surgery and hypoglossus-facial anastomosis.
* Patients submitted to recent hypoglossal-facial anastomosis.
'Patient submitted to radiotherapy.
PFP= peripheral facial paralysis; SV= Vestibular Schwannoma; FB= Fibrosarcoma; ID= Idiopathic;
FAF= Firearm wound.
* Classification of facial nerve recovery, based on the criteria by HOUSE &BRACKMANN (1985).
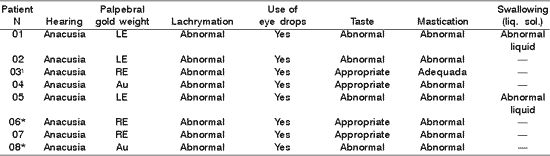
Table 2. Distribution of 8 Patients with PFP submitted to HFA concerning hearing, gold weight, lachrymation, use of eye drops, taste, mastication and swallowing.
* Patients submitted to recent hypoglossal-facial anastomosis.
LE= left eye; RE= right eye; Au- absent; - = data not referred by the patients.
¹Patient submitted to radiotherapy.
Table 3. Distribution of 8 patients with PFP submitted to HFA concerning facial symmetry at rest.
*Patients submitted to recent hypoglossal-facial anastomosis.
¹ Patient submitted to radiotherapy
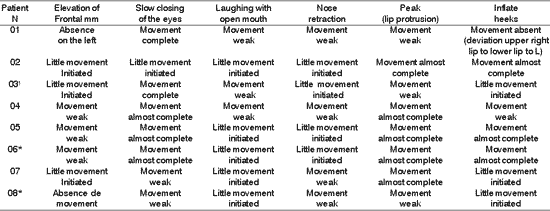
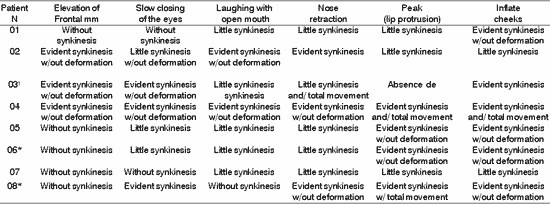
Table 4. Distribution of 8 Patients with PFP submitted to HFA concerning facial voluntary movement.
*Patients submitted to recent hypoglossal-facial anastomosis.
¹ Patient submitted to radiotherapy
Sup.= superior; Inf - inferior; D - right; E= left.
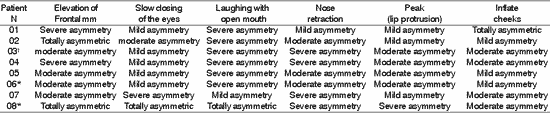
Table 5. Distribution of 8 Patients with PFP submitted to HFA concerning facial symmetry during voluntary movement.
*Patients submitted to recent hypoglossal-facial anastomosis.
¹ Patient submitted to radiotherapy
Sup.= superior; Inf - inferior; D - right; E= left.
TABLE 6. Distribution of 8 Patients with PFP submitted to HFA concerning facial synkinesis in movement.
*Patients submitted to recent hypoglossal-facial anastomosis.
¹ Patient submitted to radiotherapy
Sup.= superior; Inf - inferior; D - right; E= left.
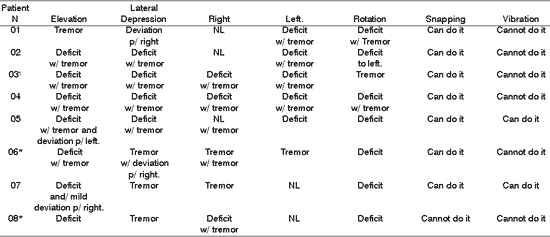
Table 7. Distribution of 8 Patients with PFP submitted to HFA concerning tongue motricity
*Patients submitted to recent hypoglossal-facial anastomosis.
¹ Patient submitted to radiotherapy
Right; Left , NL= normal.
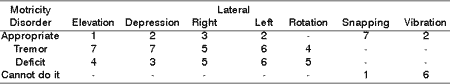
Table 8. Distribution of tongue motricity disorders in 8 patients with PFP submitted to HFA concerning facial nerve grade function.
Table 9. Distribution of tongue motricity disorders in 8 patients with PFP submitted to HFA concerning type of tongue movement.
Table 10. Distribution of facial symmetry at rest in 8 patients with PFP submitted to HFA concerning grade of paralysis.
DISCUSSION
The most frequent etiology of facial paralysis was exeresis of vestibular nerve Schwannoma. The duration of the facial paralysis and age of patients did not influence the final result of facial mimics, but influenced negatively the function of speech structures, hindering conversation skills because of tongue atrophy 3.
In the case of the patient with fibrosarcoma, since he was submitted to radiotherapy, the improvement was slow and the functional result of facial mimics was poor. We did not find in the literature any studies relating fibrosarcoma post-exeresis and radiotherapy related to HFA. We believe that maybe the duration of facial paralysis (6 years), the age of the patient and the effect of radiotherapy could be factors to explain the existence of the fibrosis in the treated region, impairing the myofunction of the respective organs.
In Table 1 all patients were submitted to HFA after onset, and duration of PFP ranged from 1 to 6 years, being that 4 of them had it in the first year. Kessler et al. referred that the interval between installation of PFP and HFA was between the 5th and 21st months. Sawamura et al. conducted HFA in the interval between the 20th and 45th months.
In Table 2, 6 patients had indication of gold weight (1 gram) in an attempt to favor eyelid closure and corneal lubrication, which agrees with literature data 9, 10, 11, which validated the use of gold weight with dynamic support of eyelid closure and fight against corneal problems, resultant from lack of lubrication. In addition, they complemented about the importance of using lubricants, in order to maintain ocular hydration, which also agrees with our findings in which all patients needed to continuously use eye drops 3, 10.
As to neuro-vegetative functions, in Table 2, the questionnaire used showed that 4 out of 8 patients presented taste abnormalities, 7 presented mastication affection revealing difficulties in tongue mobility during manipulation of solid foods, requiring manual support to remove residues from the jugal-gengival sulcus and 2 patients had abnormalities followed by food leak through the labial commissure. Moreover, 2 patients referred difficulties in swallowing, with frequent choking for liquid diets or their own saliva, resultant from tongue hemi-atrophy. Despite the severe functional incapacity of the tongue, apparently there were no difficulties for mastication and swallowing of liquid foods 8, and the final result of HFA was a success in patients with long staging of PFP, which did not result in tongue functional impairment 7. However, there may be reports of difficulties in mastication after the surgery 3, and our data did not agree with these authors.
The inconvenience of feeding after section of the hypoglossus nerve, especially in the 2nd and 3rd months after HFA, were reported by our patients 4, such as lip, tongue and cheek mobility 11. In our sample, abnormal functions of mastication and swallowing were detected, in agreement with the literature, even beyond the duration of HFA reported by them. We did not find in the literature reference to abnormal taste and presence of choking with liquid foods after HFA, as demonstrated in Table 2, which can be related to the section of innervation of the 12th nerve (hypoglossal loop).
As to facial symmetry at rest, we observed in Tables 3 and 10 that nasolabial fold was less evidenced, the presence of eyelid drop and incomplete eye closure for grades III, IV and V, which were not observed for grade II. The inappropriateness of mouth angle alignment present in grades VI and V was not observed in grades II and III.
In the literature, successes such as facial muscle tone restoration, non-labial field symmetry, appropriate and symmetric eyelid closure and improvement in alignment of the mouth angle, were found after HFA 8, 7. Even in patients that had performed HFA for a long time, we did not observe so much improvement as reported in the literature. It may be justified by the insufficient number or the reduced innervation of axon fibers 12. However, in our study, we found only one patient grade II of PFP that presented such structures appropriate at rest.
The presence of symmetry shown in Table 4, affections of voluntary muscles shown in Table 5 and the presence of synkinesis shown in Table 6 for the functions of frontal muscle elevation, slowly closure of the eyes, open mouthed laughing, elevation of the nasal ala, lip protrusion and cheek inflation were difficult to execute in most patients, with varying grades and duration after HFA. The mobility of facial mimic muscles had movements that responded in mass and with dysfunction 7, 13. Synkinesis during the palpebral closure associated with the elevation of the mouth angle was observed in some patients, such as for lingual movements associated with facial mimic movement 8. We observed that facial synkinesis was evident during labial motricity, for example, in the smile favored by zygomatic muscle contraction (greater and lesser) during elevation of upper lip, complementing the stimulation of the eye orbicular movement, elevating the lower eyelid and reducing the eyelid opening, simultaneously. In addition, the functions of lip protrusion and cheek inflation, uni or bilateral, also resulted in great level of impairment during movement, providing asymmetry of varied grades.
However, upon asking the patients to elevate the tongue up to the incisor papilla, the synkinesis was less evident compared to the tongue in rest on the mouth floor.
When the patient is submitted to HFA, it is expected that some nervous fibers of the tongue function as facial mobility, both in HFA early or recent, even considering that the movements of lingual elevation are compromised by tongue hemi-atrophy.
As to elevation, depression, lateralization, rotation and tongue vibration, Tables 7, 8 and 9 demonstrated that a large part of the patients presented deficit movements with the presence of tremor, deviations and difficulties in performing them. Such findings are in agreement with the literature, which addresses tongue movement and execution of speech functions, mastication, and swallowing present in tongue hemi-atrophy 5.
During speech, mastication and swallowing the participation of tongue mobility is essential. To that, there is the need for having anatomical and physiological integrity. In the presence of HFA, there is impairment of tongue innervation, favoring atrophy, deviation and tremors during movement, in addition to permanent numbness in greater or lower grade, reported by all patients, demonstrated in Tables 7, 8, and 9.
The sounds of Portuguese language are produced by placing the structures (lips, tongue and cheeks) in their correct positions and articulation regions. The tongue participates in a wide range of phonemes during the mobility associated with the lips, such as for example the lingo-dental phonemes /t/, /d/, /l/ and /n/; lingo-palatal phonemes /lh/, /nh/; lingo-velar phonemes /k/, /g/ and lingo-alveolar phonemes /i/, /r/ and /rr/.
As to swallowing, the oral preparation stage, mastication of the food bolus occurs with the participation of the tongue. However, the presence of hemi-atrophy and mobility affection occurs in stasis of the oral vestibule on the facial paralysis side, hindering mouth cleaning. Next, swallowing can also be impaired owing to reduction of the movement of propulsion and ejection of the bolus from the transition between the oral phase to the pharyngeal phase, for foods of varied consistency. Specially for the liquid phase, there can be lateral leak and, together with mobility and tongue hemi-atrophy, the patient can present choking, which can be related to the sectioning of the 12th nerve (hypoglossus loop). Such alteration was reported in 2 patients, which led to a picture of HFA-related dysphagia.
In the questionnaire we used, all patients revealed difficulties in the studied functions. However, tongue atrophy did not show hindrance of the movements of tongue snapping and vibrating.
CONCLUSION
Despite the clinical functional improvement of the face, we observed the results and concluded that:
1. The expectation of PFP improvement after HFA was lower than expected by patients;
2. There was articulation impression as a result of tongue atrophy;
3. There was affection of the mastication function especially for solid foods, even in patients operated a long time before;
4. The affections of posture and lip mobility hindered or reduced the absence of intra oral pressure in the oral phase of swallowing, favoring the occurrence of oral leak of liquid consistency foods.
5. There was affection of swallowing, especially for liquid foods.
REFERENCES
1. Fisch U. Current surgical treatment of intratemporal facial palsy. Clinics in Plastic Surgery 1979;6:377-88.
2. Alfordb R, Chwaber MK. Eletrical excitability tests should serve as useful measures to determine the need for descompression of the facial nerve. In: Snow JB. Controversy in Otolaryngology. Philadelphia W.B.: Saunders Company; 1980. p. 134-6.
3. Arai H, Sato K, Yanai A. Hemihypoglossal-facial nerve anastomosis in treating unilateral facial palsy after acoustic neurinoma resection. J Neurosurg 1995;82:51-4.
4. Chang CGC, Shen AL. Hypoglossal-facial anastomosis for facial palsy after resection of acoustic neuroma. Surg Neurol 1984;21:282-6.
5. Linnet J, Madsen FF. Hypoglossal-facial nerve anastomosis. Acta Neurochir (Wuen) 1995;133:112-5.
6. House J, Brackmann D. Facial nerve grading systems. Otolaryngology Hear and Neck Surgery 1985;93:146-7.
7. Sawamura Y, Abe H. Hypoglossal-facial nerve side-to-end anastomosis for preservation of hypoglossal function: results of delayed treatment with a new technique. J Neurosurg 1997;86:203-6.
8. Kessler LA, Moldaver J, Pool JL. Hypoglossal-facial anastomosis for treatment of facial paralisys. Neurol 1959;9:118-25.
9. Gavron JP, Clemis JD. Hypoglossal-facial nerve anastomosis: a review of forty cases caused by facial nerve injuris in the posterior fossa. Laringoscope 1984;94:1447-50.
10. Terziz JK. Eye sphincter substitution schemes. Eur Arch Otolaryngol Suppl 1994;S:151-5.
11. Hammerschlag PE. Facial reanimation with jump interpisitional graft hypoglossal facial anastomosis and hypoglossal facial anastomosis: evolution in management of facial paralysis. Laryngoscope 1999;109 (Suppl.90):1-23.
12. May M, Sobe SM, Mester SJ. Hypoglossal-facial nerve interpositional jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg 1991;204:818-26.
13. Rosenwasser RH, Liebmen E, Limenez F, Buchheit WA, Andrews DW. Facial reanimation after facial nerve injury. Neurosurg 1991;29:568-74.
1 Speech and Voice Therapist, Specialist in Human Communication Disorders, Federal University of São Paulo (UNIFESP/EPM).
Master studies in Neuromotor Rehabilitation Sciences under course, Universade Bandeirantes de São Paulo- UNIBAN.
2 Ph.D., Affiliated Professor, Discipline of Pediatric Otorhinolaryngology, Federal University of São Paulo, responsible for the unit of Facial Nerve Disorders (UNIFESP/EPM).
3 Ph.D., Associated and Full Professor, Discipline of Otorhinolaryngology, Federal University of São Paulo (UNIFESP/EPM).
Study conducted at the Discipline of Otorhinolaryngology, Department of Otorhinolaryngology and Human Communication Disorders, UNIFESP/EPM.
Address correspondence to: Elisabete Cardoso Coelho Ferreira da Silva - Rua Teixeira de Mello 221 ap. 032 Tatuapé São Paulo 03067-000
Tel (55 11) 6198-5382 / 9181-7700 - E-mail: Elisabete_silva@hotmail.com
Study presented as free communication, candidate for an award at 35º Congresso Brasileiro de Otorrinolaringologia -
9º Congresso Iberolatinoamericano de Otorrinolaringologia 2000 Natal/RN, Brazil.
Article submitted on July 26, 2002. Article accepted on May 13, 2003.