Year: 2004 Vol. 70 Ed. 1 - (17º)
Artigo de Revisão
Pages: 106 to 111
Adverse effects of topical and sistemic medications in the oral mucosa
Author(s):
Caio C. S. Loureiro 1,
Carlos A. Adde 2,
Flávio E. G. Perez 2,
Sibele S. Penha 2
Keywords: mouth mucosa, adverse effects, administration, topical, drug toxicity
Abstract:
Many of the commonly used with or without medical prescription drugs potentially cause adverse reactions in the oral mucosa. Thus, it is necessary that health care providers become conscious about the possible undesired effects caused by each medication, to enable an adequate clinical conduct in order to take the necessary steps to minimize them. In this paper the authors present a review of the drugs that most commonly cause these alterations.
![]()
INTRODUCTION
Patients submitted to routine dental follow up can occasionally require indication of clinical therapy support drugs, or can make use of specific drugs, either prescribed or over-the-counter. Considering that many of the side effects of drugs manifest in the oral mucosa, physicians and dental surgeons should know how to recognize them. We tried to highlight the most common clinical manifestations reported by the literature as being the most prevalent, to wit: mucosa inflammatory reactions, ulcerations, gingival hyperplasia and pigmentation.
Despite the fact that oral mucosa reactions to the use of drugs is relatively common, its varied clinical aspects can sometimes hinder early etiologic diagnosis.
Patients that develop intraoral signs or symptoms that can be attributed to drug therapy should look for healthcare support, and professionals evidently have to be able to recognize them, get to know what are the commonly involved drugs and try to establish a potential correlation between drugs and oral adverse events.
Appropriate anamnesis, including medical complete history is essential for the professional to be able to make the diagnosis of affections and conclude for the most appropriate treatment to solve the problem.
In this review, the authors describe a variety of chemical agents and drugs that can produce these side effects in the oral cavity, according to the type of clinical manifestation or clinical aspect, grouping the drugs that cause similar adverse reactions.
INFLAMMATORY REACTIONS
Stomatitis
One of the most common drug reactions is stomatitis or oral mucosa inflammation. Most of the cases of stomatitis induced by drugs manifest nothing more than erythema, normally followed by pain. In cases with clinical and/or etiology specific characteristics, it is possible to subclassify stomatitis into allergic, lichen eruption and multiform erythema.
Stomatitis may result from the use of anti-neoplastic drugs, allergy to drug or direct contact between the drug and the mucosa.
Anti-neoplastic and chemotherapy drugs currently use are one of the most common causes of generalized non-specific stomatitis 1, 2. These drugs have been used with increasing frequency as adjuvant therapy with surgery and/or therapy for the treatment of some localized malignant diseases.
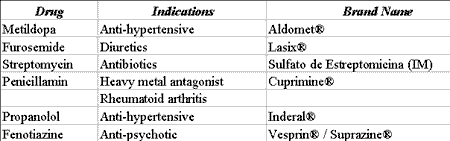
Forty percent (40%) of the patients that use chemotherapy agents for the treatment of cancer develop some type of oral complication as a result of treatment. Cytotoxic drugs such as methotrexate, 5-fluorouracil, 6-marcaptopurane, clorambucil, dexorubicin and bleomycin destroy the mucosa cells as a result of inhibition of epithelial mitosis, inducing atrophy and providing development of stomatitis and spontaneous or traumatic ulcerations (Table 1). This picture is frequently manifested 4 to 7 days after the beginning of chemotherapy treatment and is normally characterized by generalized painful erythema 3.
Most of the patients in chemotherapy treatment develop stomatitis during hospitalization. Treatment is normally palliative comprising mouth washing with antiseptic solution and topical anesthetic drugs. More severe cases may require additional prescription of analgesics and antimicrobials to prevent secondary infection.
Allergic stomatitis
Many different drugs prescribed systemically or locally can cause hypersensitivity or allergic reactions. The large number of compounds considered to be allergenic makes it difficult to create a complete list. Eversole4, in 1979, presented a review about the mouth allergic reactions, referring to common allergenic substances such as topical drugs, antibiotics (specially penicillin), sulfa, nickel, additives to mouth-washing solutions and tooth pastes, soaps, among others.
Allergic reactions occur locally by the contact between the mucosa and the allergen or result from systemic administration of the drug 5. A localized inflammatory area that got in contact with the allergen or even a more generalized area owing to the use of moth-washing solution, for example, can be named localized allergic stomatitis.
Drug-induced stomatitis, a systemic reaction, is presented as a diffuse reaction that can be associated with a clinical picture of rash 4. In most of the cases, manifestation is limited to the oral cavity, but there can be cases of perioral rash as well. The medical history of the patient is important to identify risk patients, since oral cavity allergic reactions are more common in patients with reports of previous allergic processes 6.
Inflammatory reaction normally starts with symptoms such as burning sensation or tingling, followed by erythema and formation of blisters. The most severe reactions can evolve to ulcerations. Normally, this process occurs hours after the contact with the allergen.
The correct identification of the allergen can be difficult and require extensive clinical history and occasionally the conduction of daily record of everything the patient introduced in the oral cavity. After the identification of the allergen, confirmation can be achieved through skin tests 6. The treatment of allergy requires elimination of allergens and, if symptoms are more severe, the administration of specific drugs may be necessary.
Lichen eruptions to drugs
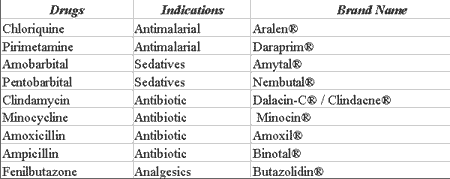
Lichen planus is an autoimmune disease that affects the skin and/or oral mucosa. It is clinically characterized by reticular striae or Wickham's striae (similar to white plaques), which can develop areas of erosion and ulceration and is frequently associated with emotional disorders 7, 8. Many drugs can cause an indistinguishable reaction to lichen planus. This reaction is named lichen eruption to drugs and presents characteristics similar to lichen planus, frequently with erythematous lesions 9. Most of the drugs associated with this reaction are presented in Table 2.
All patients with lichen planus should be questioned whether they had recently used drugs. The reaction normally disappears when the drug intake is interrupted.
Multiform erythema
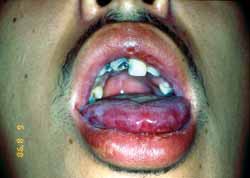
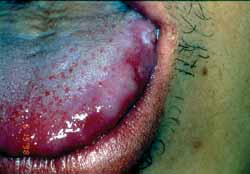
Multiform erythema is a mucous-cutaneous immune reaction normally observed after viral infections, being that the most common is caused by simple herpes virus 7, 10. Cases associated with drug treatment have also been documented. After administration of certain drugs, there are normally characteristic signs such as fever, malaise, myalgia, arthralgia, and sometimes vomiting. The patient starts to present acute mucous-cutaneous eruptions. Blisters and skin ulcerations are normally present. Normally, there is involvement of palm-plantar regions. Oral involvement is common and characterized by symmetrical blister-bullous erythematous reaction (Figures 1 and 2). There is a trend to involve the lips and we commonly see the formation of crusts following erosion of the lesion. The most severe form of multiform erythema with extensive involvement of the skin, eyes, and genital organs is known as Stevens-Johnson Syndrome7.
Among the drugs described and associated with multiform erythema, we can include antimalariall agents, barbiturates, some antibiotics such as clindamycin, tetracycline, and penicillin, and some antiinflammatory analgesics (Table 3).
The diagnosis of multiform erythema is essentially clinical, and the drug should be interrupted. Mild cases can be treated symptomatically. More severe reactions normally respond well to systemic administration of cortical steroids, which may require hospitalization.
ULCERATION AND NECROSIS
Systemic as well as topical drugs can lead to ulcerated lesions in the oral mucosa. Many chemicals, when in contact with the soft tissue of the mouth mucosa, can cause necrosis of the epithelium surface, especially when inappropriately used by the patients.
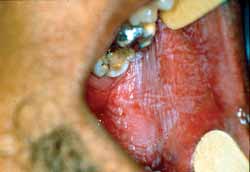
One of the most common drugs that is incorrectly used in topical presentation is aspirin: the pill is sometimes mistakenly placed on the mucosa to relieve pain. The prolonged contact of the medication with the oral mucosa can cause necrosis of the epithelium resulting in the classical so-called aspirin burn, in which the affected tissue becomes whitish and, depending on severity of the impairment and tissue destruction, it may lead to painful and bleeding area (Figure 3).
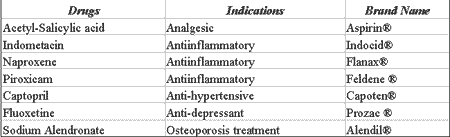
Other systemic drugs, even when administrated correctly, can trigger the onset of oral cavity ulcers 11. Some antiinflammatory 12, anti-hypertensive 13, antidepressants and drugs used to treat osteoporosis 14, can occasionally cause these affections (Table 4).
This effect is normally a result of overdosage, requiring adjustment of the doses and sometimes drug replacement.
GINGIVAL HYPERPLASIA
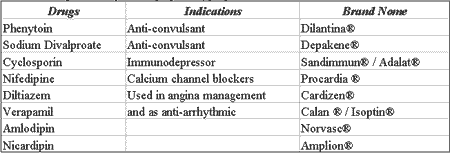
Gingival hyperplasia is the increase in gingival volume resulting from exacerbated cell proliferation, which can be caused by the use of specific medications 15. One of the drugs that has been used for a long time and causes this side effect is phenytoin (Dilantin) 26 used in the treatment of patients with epilepsy seizures. Even though the action mechanism through which phenytoin would cause gingival hyperplasia has not been properly defined yet, it is present in about half of the patients that take it. In initial stages, the gingival increase is characterized by non-painful growth of the interdental papilla detected about 2 to 3 months after use of the drugs. The growth of gingival tissue can continue for about one year and in more severe cases it covers the patient completely. This effect is normally not dose-dependent and duration-dependent, but it depends rather on the degree of local irrigation and inappropriate oral hygiene.
Gingival increase is translated by fibrous hyperplasia, which is clinically characterized as firm tissue that distinguishes it from the edema caused by inflammation or leukemic infiltrate. Since firm and fibrous gingival hyperplasia can also be found in many hereditary syndromes, differential diagnosis with leukemia and other conditions that present similar inflammatory characteristics is extremely important and should be quick and precise.
Other anti-epileptic agents such as sodium valproate 17 and phenobarbital are also gingival hyperplasia causing agents.
In addition to anti-convulsing agents, other drugs have been responsible for gingival tissue hyperplasia. Among them, we can include cyclosporin, frequently used immunodepressant agents after organ transplantation, which cause gingival hyperplasia in about 30% of the patients and powerful calcium blocker agents used in the treatment of angina and antiarrhythmic drugs 18-21.
Gingival hyperplasia secondary to drugs referred above is similar both clinically and histologically, suggesting a common action mechanism. Since all these drugs inhibit the inflow of calcium through the plasma membranes, it is believed that since they change the intracellular level of calcium, these drugs can reduce the activity of collagenase, resulting in excessive synthesis of collagen and fibroblast proliferation in the gums 22.
The treatment includes discontinuation of the drug, gingivectomy and implementation of the oral hygiene procedures. A careful oral hygiene program started together with drug administration can reduce the incidence of this adverse effect. Normally, surgical treatment of hyperplasia presents better results, even though recurrence is common in the absence of strict oral hygiene. Even knowing that phenytoin induces folate deficiency there is preliminary evidence that administration of folic acid can inhibit or revert tissue growth in some patients 9. To severe cases, the prescription of another anti-convulsing drug should be considered.
Table 5 shows the main drugs that can trigger a hyperplastic process on the oral mucosa.
PIGMENTATION
Pigmentation of soft tissues in the oral cavity can be of physiological or pathological nature 23 (Addison's disease, Peutz-Jegher syndrome, Albright's disease, neurofibromatosis, among others) or of endogenous or exogenous origin. The history of pigmentation disorders, as well as investigation of family history, use of drugs, duration, staining and distribution of lesions is important for differential diagnosis.
Exogenous pigmentation normally results from traumatic implementation of materials such as amalgam and lead pencil whereas endogenous pigments are resultant from red blood cells destruction or increased production of melanin. As to oral pigmentation resulting from drug therapy, systemic administration for prolonged periods of time are responsible for the onset of lesions and diffuse areas of multifocal pigmentation.
Heavy metals such as lead, mercury and bismuth when ingested or inhaled can potentially cause linear pigmentation along the gingival rim. This pigmentation is directly related to inflammatory processes, which increase the vascular permeability allowing deposition of metals in the gingival cervical margins through precipitation with sulfites released by local bacteria 24.
Drugs used as antimalarial 25, such as chloriquine, pirimetamine, quinine, quinacrine and quinidine, which are also used for the treatment of lupus erythematous and rheumatoid arthritis, can cause the onset of macular lesions with well-delimited rims of oral mucosa 26.
Other drugs that cause oral pigmentation include phenolphthalein, the group of fenotiazines and cytotoxic drugs such as anti-neoplastic agents.
Pigmentation of perioral skin (cloasm) and intraoral pigmentation can be observed after use of oral contraceptives 27.
Pilous black tongue is a frequent picture that can occur after the use of drugs such as sodium perborate, sodium peroxide, large spectrum antibiotics used for a long period of time and corticosteroids 26.
HIV positive patients that use azidotimidine can present circumscribed or diffused macular lesions in the oral mucosa. These lesions, in some cases, are recurrent lesions after surgical excision 28.
Tetracycline antibiotics, especially minocycline 29, 30, have as adverse effect the onset of pigmented skin on dorsal mucosa lesions. In addition to soft tissue pigmentation, this class of drugs can also affect the structure and cause staining in deciduous and permanent teeth.
Chlorexidine, an anti-bacterial agent that reduces the formation of plaques, normally causes teeth staining and can induce oral mucosa pigmentation.
Table 6 shows the main drugs related to pigmented lesions and oral mucosa staining.
CONCLUSIONS
As a result of the great variation in adverse events manifested in intraoral soft tissues, it is important that health care professionals recognize these abnormalities and try to observe cause-effect relation based on well-conducted and comprehensive clinical history.Table 1. Drugs that may cause stomatitis.
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Table 2. Drugs that may cause lichen eruptions.
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Table 3. Drugs that may cause multiform erythema.
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Table 4. Drugs that may cause ulcerations and necrosis.
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Table 5. Drugs that may cause gingival hyperplasia
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Table 6. Drugs that may cause pigmentation.
Source: DICIONÁRIO DE ESPECIALIDADES FARMACÊUTICAS: DEF 2002/03
Figure 1. Clinical aspects of multiform erythema.
Figure 2: Clinical aspects of multiform erythema.
Figure 3. Clinical aspect of "aspirin burn" in the jugal mucosa.
REFERENCES
1. Cherubini K, Figueiredo MAZ, Lorandi CS, Yurgel LS. Reações adversas a drogas. RGO Jan,1994; 42(1):34-6.
2. Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol Jan 1998; 34(1):39-43.
3. Kane M, Zacharczenko N. Oral side effects of drugs. NYSDJ Jan 1993; 59(1)37-40.
4. Eversole LR. Allergic Stomatitides. J Oral Med Oct, 1979; 34(4): 93-102.
5. Smith RG, Burtner AP. Oral side effects of the most frequently prescribed drugs. Spec Care Dentist Mar 1994; 14(3):96-102.
6. Tosti A, Piraccini BM, Peluso AM. Contact ands irritant stomatitis. Semin Cutan Med Surg Dec. 1997; 16(4):314-9.
7. Axell T. Hypersensitivity of the oral mucosa: clinics and pathology. Acta Odontol Scand Oct 2001; 59(5):315-9.
8. Hampf BG, Malmstrom MJ, Aalberg VA, Hannula JA, Vikkula J. Psychiatric disturbance in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol 1987; 63:429-32.
9. Backman N, Holm AK, Hanstrom L, Blomquist HK, Heijbel J, Safstrom G. Folate treatment of diphenylhydantoin-induced gingival hyperplasia. Scand J Dent Res Jun, 1989; 97(3):222-32.
10. Machado ACP, Silveira FRX, Sugaya NN, Birman EG. Eritema multiforme: revisón de interés en Estomatología. Rev Fola/Oral Feb 1997; 3(7):19-24.
11. Madinier I, Berry N, Chichmanian RM. Drug-induced oral ulcerations Ann Med Interne Jun 2000 (Paris); 151(4):248-54.
12. Siegel MA, Balciunas BA. Medication can induce severe ulcerations. J Am Dent Assoc Sep 1991; 122(10):75-7.
13. Boulinguez S, Bonnetblanc JM. Aphthae or painful ulcers induced by nicorandil. Presse Med Nov 2000; 29(33):1828-32
14. Gonzalez-Moles MA, Bagan-Sebastian JV. Alendronate-related oral mucosa ulcerations. J Oral Pathol Med Nov 2000; 29(10):514-8.
15. Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol Mar 1996; 23(3 Pt 1):165-75.
16. Desai P, Silver JG. Drug-induced gingival enlargements. J Can Dent Assoc Apr 1998; 64(4):263-8.
17. Anderson HH, Rapley JW, Williams DR. Gingival overgrowth with valproic acid: a case report. ASDC J Dent Child Jul-Aug, 1997; 64(4):294-7.
18. Harel-Raviv M, Eckler M, Lalani K, Raviv E, Gornitsky M. Nifedipine-induced gingival hyperplasia. A comprehensive review and analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod Jun1995; 79(6):715-22.
19. Infante Cossio P, Torello Iserte J, Espin Galvez F, Garcia-Perla A, Rodriguez-Armijo Sanchez L, Castillo Ferrando JR. Gingival hyperplasia associated with amlodipine. Ann Med Interna Feb 1997; 14(2):83-5.
20. Nohl F, Ferrari P. Drug-induced gingival hyperplasia. Ther Umsch Sep 1998; 55(9):573-5.
21. Pascual-Castroviejo I, Pascual Pascual SI. Nicardipine-induced gingival hyperplasia. Neurologia Jan 1997; 12(1):37-9.
22. Butler RT, Kalkwarf KL, Kaldahl WB. Drug-induced gingival hyperplasia: phenytoin, cyclosporin and nifedipine. J Am Dent Assoc 1987; 144:56-60.
23. Lenane P, Powell FC. Oral pigmentation. J Eur Acad Dermatol Venereol Nov, 2000; 14(6):448-65.
24. Rogers SN, Vale JA. Oral manifestations of poisoning. Brit Dent J Feb 1993; 174(4): 141-3.
25. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod Aug 2000; 90(2):189-94.
26. Wright JM. Manifestações bucais das reações das drogas. In: Clínicas Odontológicas da América do Norte. Farmacoterapia. Trad. Catach C, Gutemberg C. São Paulo: Roca; 1985. Cap.10, p.149-164.
27. Dicionário de Especialidades Farmacêuticas: DEF 2002/03 -31ed.- Rio de Janeiro: Ed. de Publicações Científicas; 2002.
28. Ficarra G, Shillitoe EJ, Adler-Storthz K, Gaglioti D, Di Pietro M, Riccardi R, Forti G. Oral melanotic macules in patients infected with human immunodeficiency virus. Oral Surg Oral Med Oral Pathol Dec 1990; 70(6):748-55.
29. Cheek CC, Heymann HO. Dental and oral discolorations associated with minocycline and other tetracycline analogs. J Esthet Dent 1999; 11(1):43-8.
30. Ozog DM, Gogstetter DS, Scott G, Gaspari AA. Minocycline-induced hyperpigmentation in patients with pemphigus and pemphigoid. Arch Dermatol Sep, 2000; 136(9):1133-8.
1 Dentist - Intern, Discipline of Integrated Clinical Practice - Department of Stomatology, Dental School, USP - FOUSP.
2 Ph.D., Professor, Discipline of Integrated Clinical Practice - Department of Stomatology, Dental School, USP - FOUSP. Dr. Caio César de Souza Loureiro
Dental School - Dept. Stomatology - Disc. Clínica Integrada - Av. Prof. Lineu Prestes, 2227 Cidade Universitária 05508-900 Sao Paulo SP
E-mail: dr_caio@hotmail.com