Year: 2004 Vol. 70 Ed. 1 - (3º)
Artigo Original
Pages: 24 to 28
Correlation of the salival pH and volume with laryngeal-pharyngeal symptoms
Author(s):
Henrique Olival Costa 1,
Cláudia Alessandra Eckley 2
Keywords: laryngitis, saliva, diagnosis, pH, volume
Abstract:
In spite of the great enthusiasm wakened up by the progress of the concepts of the denominated syndrome of the larygopharyngeous reflux (LFR), it is still difficult for the otorrinolaringologist to establish with safety if this illness can be considered in the future as a disease similar to the gastro esophageal reflux. The inflammatory signs in the segment, found in some patient that present phmetry with pH below 4 in the proximal probe can be found in other situations aggressive to the area, not being, therefore, patognomonic of LFR. The conditions of saliva, the volume, clearence and alterations of the eletrolitic conditions can influence the capacity of protection of the regional mucous membrane. Aim: To observe the relationship between the inflammatory symptoms and the pH and volume of saliva. Study Design: Observacional cohort with transversal cut. Material and Method: 59 subjects were studied, with ages varying from 24 to 76 years, with average 50,5 years, being 44 women and 15 men. All the patients answered to a questionnaire on laryngopharyngeous symptoms and they had his/her saliva collected and measured its volume and pH. Results: The median volume of saliva collected in the patients was 4,3 ml, with the minimum of 1,5 and a maximum of 7,5. The median pH was of 7,1, extending from 6 to 8. Of the total of symptoms 31 patients presented dysphonia, 39 throat clearing, 2 dyspnea, 24 halitosis, 4 caseum, 4 recurrent tonsillitis, 6 teeth and gingival problems, 9 oral ulcers, 6 xerostomia, 12 glossodynia, 36 globus pharingeus, 2 odinophagia and 8 dysphagia. The several correlations among pH, volume to salivate and symptoms were observed showing, in some cases, strong positive or negative correlation. Conclusion: The pH of saliva, in the dependence of its volume can have strong interference in the laryngopharyngeous inflammatory symptoms.
![]()
INTRODUCTION
Despite the great enthusiasm triggered by the advance of concepts that defined laryngopharyngeal reflux (LPR), it is still difficult for otorhinolaryngologists to safely define whether this syndrome will be considered in the future a disease similar to gastroesophageal reflux 1. In our opinion, inflammatory signs of laryngopharyngeal segment found in some patients that present 24-hour pH monitoring test with pH below 5 in the proximal probe can be found in other situations that affect the region, and they are not pathognomonic of LPR. Among the possibilities of local aggression we can include hyperacidity caused by cigarette inhaling, intake of caustic foods or temperature extremes and gingival, nasal or oropharyngeal infections 2-6. In addition to these facts, the conditions of salivary flow, volume, clearance and electrolytic saliva substances can influence the capacity of regional mucosa protection 7-12.
The balance between these aggression factors and pharyngolaryngeal protection elements have been studied in many different aspects, be it by measuring epithelial factors such as protein expression (TNF, EGF and p53), be it by substances that may contribute to tissue aggression such as pepsin, hydrochloric acid and presence of fungus and bacteria 13-16.
Knowing that saliva is one of the main responsible elements for maintenance of oral homeostasis, contributing to pH and oral flora stability, the knowledge of the correlation between salivary pH and its volume with laryngopharyngeal symptoms associated with LPR is an extremely important factor in the clinical practice, both therapeutically and for the diagnosis of inflammatory affections of the segment.
To present, little attention has been directed to recognizing what is the optimal saliva composition and which changes are determined by factors such as level of hydration, food, exercise and emotional status. This knowledge would be important if saliva, in fact, played a role in regional homeostasis and could be determinant in the production of local signs and symptoms. To clarify it, Chicharro et al.17 studied the effects of physical activity in salivary composition. According to the authors, exercise can induce changes of various salivary components such as immunoglobulins, hormones, lactate, proteins and electrolytes. The authors considered that saliva composition would be used as an indicator of body tissues responses to different physical efforts. Therefore, the response of amylase and salivary electrolytes for the increment in physical activity would be of particular interest. According to the studies, in addition to exercise intensity, and coinciding with accumulation of blood lactate, there is clear increase in alpha-amylase and electrolyte salivary levels (specially Na+).
Interestingly, some drugs used to treat patients with laryngopharyngeal symptoms caused by gastroesophageal reflux can also cause salivary composition affections. Among them, cisapride, a prokinetic drug, has shown its capacity to increase salivary volume in some studies, which, consequently, increases acid-protection capacity. Chen et al.18 studied 55 patients with reflux esophagitis by scintigraphy. They divided the sample into two groups, in one group they maintained the patients fasting and offered cisapride and in the second group, they fed the patients and offered cisapride. Salivary flow was studied before and after drug administration. There was significant increase in salivary volume in subjects that received cisapride after feeding.
Proton pump inhibitors may have effects on saliva production. Mechanisms of primary fluid formation through the mandibular glands of red kangaroos that used ion-transport and carbonid anhydrase inhibitors in salivary concentrate were investigated. After intake of butesamide, kangaroos simultaneously presented decrease of [Na], [Cl] and osmolarity, in addition to increase in composition of [K] and [HCO3]. Animals received bumetanide associated with amyloride, there was increase of [K] and [HCO3] and reduction of [Cl], without any effect on [Na] or total salivary flow19.
As we can see, there are different implications in the capacity of buffering and in the protective action that saliva has when submitted to conditions that have not been completely studied yet. These conditions are little known and could change the course of affection as prevalent as laryngopharyngeal reflux if they were properly understood and submitted to the appropriate manipulation.
OBJECTIVE
The purpose of the present study was to observe the correlation between laryngopharyngeal symptoms of inflammation and pH and salivary volumes.
MATERIAL AND METHOD
We studied 59 subjects aged 24 to 76 years, mean age of 50.5 years, being 44 women and 15 men. Patients were selected and based on a sample followed in the Ambulatory of Gastroenterology, Hospital Central da Santa Casa de Sao Paulo, and referred for assessment of the presence or absence of laryngopharyngeal reflux. All patients referred to us were included in the sample. There was no exclusion criterion, since we did not have a null hypothesis to be tested and the study proposed an intentional investigation of the correlation between pH and salivary volume and laryngopharyngeal reflux symptoms. All the 30 patients presented symptoms of distal reflux and, therefore, they were submitted to esophageal 24-hour pH monitoring, test in which we could compare the results of pH and salivary volume. Other patients were selected in the ambulatory of Otorhinolaryngology and completed the group, being observed the characteristics of gender and age in the group submitted to 24-hour pH monitoring.
All patients signed the free informed consent term approved by the Ethics Committee, Santa Casa de Sao Paulo.
All patients answered the questionnaire based on the studies that correlated LPR and symptomatology 20-22, in which the symptoms of dyspnea, dysphonia, throat clearing, cough, odynophagia, globus, aphthas, recurrent tonsillitis, glossodynia, dental-gingival problems, presence of oropharynx caseum and dysphonia were observed.
Patients had to fast for at least 8 hours before collection, with mean of 10.3h fast, being instructed not to make use of tooth paste on the material collection day. The collection was processed after the patients have washed the mouth with running water to eliminate epithelial desquamation and bacterial debris. Patients were seating, without swallowing and a glass container was placed under the lower lip. The saliva that spontaneously drooled from the mouth in the period of 15 minutes was collected through a funnel coupled to a test tube that was stored in a foam box with ice. Its total volume was measured and the collected pH volume was checked with a pH strip.
The results were submitted to correction statistical tests to assess the various possibilities of association between symptoms and pH and salivary volume.
Statistical analysis
The results of each one of the parameters was compared through analysis of simple regression for pairs X and Y based on the method of smaller square as model: Y = b0 + b1 * X + error, to check correlation between pH and salivary volume and symptoms presented. The findings of 24-hour pH monitoring were correlated with salivary pH.
RESULTS
The mean total volume of saliva collected was 4.3ml, with minimum of 1.5 and maximum of 7.5. Mean pH was 7.1, ranging from 6 to 8. Out of the total presented symptoms, 31 patients presented dysphonia, 39 throat clearing, 2 dyspnea, 24 halitosis, 4 caseum, 4 repetitive tonsillitis, 6 dental-gingival problems, 9 aphthas, 6 xerostomia, 12 glossodynia, 36 globus pharyngeus, 2 odynophagia, 16 cough and 8 dysphagia.
The results of correlation (between -1 and 1) are shown in Tables 1 and 2.
We also studied the mean saliva volume according to levels of pH and mean of pH 6 was 3.2ml, for pH 7 it was 4.1ml and pH 8 it was 4.3ml.
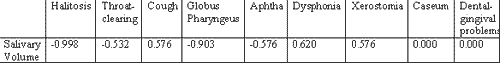
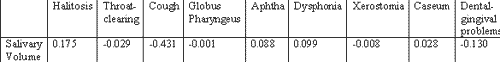
To check whether symptomatology could be correlated with saliva volume according to the pH level we observed the presence of pH symptoms versus salivary volume. The results are shown in Tables 3, 4 and 5.
DISCUSSION
As we could observe by the results, there was negative correlation between general pH value and presence of laryngopharyngeal symptomatology in the analyzed patients. We had the expectation that general salivary volume could be related with final general pH of the saliva, which did not happen. In fact, mean salivary volume was smaller in patients with lower pH (6) than in patients with higher pH (7 and 8) (3.2 vs. 4.1 and 4.3). To test the possibility of salivary volume be correlated with symptoms in accordance with pH level, we conducted a correlation test between the symptoms and volume by pH group (6, 7 and 8). The correlation was very clear for some groups, such as in the cases in which salivary pH was low and halitosis (-0.998), which means that there were no cases of halitosis that did not present salivary volume lower than the general value of the group or in the case of globus pharyngeus for the same group (-0.903). For groups of neutral or alkaline pH this correlation was less clear, but it occurred for pH 7 and cough (-0.431) and for pH 8 and dental-gingival problems (-0.442), throat clearing (-0.359) and aphtha (-0.314). These findings are in agreement with Cianci et al. who questioned whether alkaline reflux was an aggression factor. We share the same impression when we see inflammatory signs in infant rhinopharynx.
This study intended to investigate a poorly studied area that showed rich clinical potential in a condition that has great morbidity and prevalence.
Upon concluding this stage, we can suggest that the study be expanded and that new correlations be studied, such as diet type and routine, smoking, gingival and nasosinusal conditions, which are other factors that can influence regional pH and interfere with the data.
However, evidence of correlation of buffering conditions and salivary clearance with laryngopharyngeal symptoms of the so-called laryngopharyngeal reflux proved to be a local pathology and treatments that aim at changing stomach or esophageal conditions can be effective because of indirect effects on the mouth and not necessarily because of stomach alkalinization (anti-acids with H1 blockers and proton pump inhibitors)24, 25.
Recent studies revealed great interference in secretion of K, HCO3 and Cl in the saliva of rodents when ingesting proton pump inhibitors. Conversely, there is evidence that previously used drugs to improve esophageal-stomach kinetics, such as cisapride, also had effect on salivary flow, increasing its volume when taken after the meals. The confirmation that salivary pH is somewhat related with laryngopharyngeal symptoms, the dependency on the quantity of saliva produced, as well as the confirmation that higher pH occurs in subjects with greater saliva volume, and the fact that salivary pH is not directly correlated with 24-hour pH monitoring (0.164) make us see that we have a new ambulatory non-invasive diagnostic possibility to measure salivary pH and volume26. We can also start to consider new therapeutic strategies to minimize symptoms of the pathology known as laryngopharyngeal reflux.
CONCLUSION
Salivary pH, which depends on salivary volume, can have strong influence in pharyngolaryngeal symptomatology.Table 1. Results of the analysis and correlation between salivary pH and epidemiological and symptomatological factors.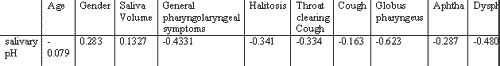
Correlation of salivary pH versus 24-hour pH monitoring was 0.164.
Table 2. Results of the correlation analysis between salivary pH and epidemiological and symptomatological factors.
Correlation of salivary pH versus 24-hour pH monitoring was -0.088.
Table 3. Result of the correlation analysis between salivary volume and symptoms in patients with salivary pH = 6.
Table 4. Result of the correlation analysis between salivary volume and symptoms in patients with salivary pH = 7.
Table 5. Result of the correlation analysis between salivary volume and symptoms in patients with salivary pH = 8
REFERENCES
1. Gavazzoni FB, De Ataíde AL, Herrero Junior F, Macedo Filho ED. Esofagite por ref1uxo e laringite por ref1uxo: estágios clínicos diferentes da mesma doença? Rev Bras ORL 2000; 68(1): 86-90.
2. Adhami T, Goldblum J, Richter J, Vaezi M. Role of gastric and duodenal ingredients in laryngeal tissue injury: an experimental study in dogs. Abstracts of the Digestive Disease Week 2002; 429: A-87.
3. Da Wes C. Circadian rhythms in human salivary flow rate and composition. J Physiol 1972, 220: 529-45.
4. Korsten MA, Rosman AS, Fishbein S, Shlein RD, Goldberg HE, Biener A. Chronic xerostomia increases esophageal acid exposure and is associated with esophageal injury. Am J Med 1991; 90: 701-6.
5. Maccini DM & Veit BC. Salivary Epidermal Growth factor in Patients with and Without Acid Peptic Disease. Am J Gastroenterol 1990; 85(9): 1102-4.
6. Benamouzig R, Ferriere F, Guettier C, Amouroux J, Coste T, Rautureau J. Role of salivary and seric epidermal growth factor in pathogenesis of ref1ux esophagitis in chronic alcoholics and nondrinkers. Dig Dis & Sci 1996; 41(8): 1595-9.
7. Moazzez R, Anggiansah A, Bartlett D, Owen W. Tooth wear saliva and symptoms of GERD: is there a relationship? Abstracts of the Digestive Disease Week 2002; Wl164: A-816.
8. Christensen ME, Therkildsen MH, Poulsen SS, Bretlau P. Transforming growth factor alpha and epidermal growth factor in laryngeal carcinomas demonstrated by immunohistochemistry. Acta Oto-Laryngol 1993; 113(4): 563-7.
9. Namiot Z, Rourk RM, Piascik R, Hetzel DP, Sarosiek J, Mccallum RW. Interrelationship between Esophageal Challenge with Mechanical and Chemical Stimuli and Salivary Protective Mechanisms. Am J Gastroenterol 1994; 89(4): 581-7.
10. Dagogo-Jack S. Epidermal growth factor EGF in human saliva: effect of age, sex, race, pregnancy and sialagogue. Scand J Gastroenterol 1986; 21 (Suppl 124): 47-54.
11. Nandurkar S, Cameron A, Fett S, Zinsmeister A, Locke III GR. Environmental causes of reflux: influence of lifestyle diet and psychological factors. Abstracts of the Digestive Disease Week 2002; S1356: A-268.
12. Gray MR, Donnelly RJ & Kingsnorth AN. Role of salivary epidermal growth factor in the pathogenesis of Barrett's columnar lined oesophagus. Br J Surg 1991; 78: 1461-6.
13. Orsini B, Brocchi A, Calabrà A, Fedip, Tommasi MS, Surrenti C. Radioimmunoassay of Epidermal Growth Factor in Human Saliva and Gastric Juice. Clin Biochem 1991; 24: 135-41.
14. Gill GA, Arthur C, Hampson F, Dettmar P, Moorghen M, Pigna Telli M. Characterization of acid and pepsin damaged laryngeal and oesophageal mucosa. Abstracts of the Digestive Disease Week 2002; Tll15: A-595.
15. Mcgurck M, Hanford L, Justice S, Metcalfe RA. The Secretory Characteristics of Epidermal Growth Factor in Human Saliva. J Oral Biol 1990; 35(8): 653-9.
16. Sarosiek J, Scheurich CJ, Marcinkiewicz M, Mccallum RW. Enhancement of Salivary Esophagoprotection: Rationale for a Physiological Approach to Gastroesophageal Ref1ux Disease. Am J Gastroenterol 1996; 110: 675-81.
17. Chicharro JL, Lucia A, Perez M, Vaquero AF, Urena R. Saliva composition and exercise. Sports Med 1998 Jul; 26(1):17-27.
18. Chen SD, Kao CH, Chang CS, Chen GH. Salivary function in patients with reflux esophagitis: effect of cisapride. J Nucl Med 1998 Aug; 39(8):1449-52.
19. Beal AM. The effect of transport-blocking drugs on secretion of fluid and electrolytes by the mandibular gland of red kangaroos Macropus rufus. Arch Oral Biol 1997 Oct-Nov; 42(10-11):705-16.
20. Costa HO, Eckley CA, Fernandes AMF, Destailleur D, Villela PH. Ref1uxo gastroesofágico: comparação entre os achados laríngeos e digestivos. Rev Port ORL 1997; 35(1): 21-6.
21. Eckley CA & Costa HO. Manifestações otorrinolaringológicas da doença do ref1uxo gastroesofágico In: Martinez JC. Atheneu Affecções Cirúrgicas do Estômago e Intestino Delgado. Sao Paulo; 2002 (in press)
22. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991; 101(Suppl): 1-78.
23. Sarosiek J & Mccallum RW. What Role do Salivary Inorganic Components Play in Health and Disease of the Esophageal Mucosa? Digestion 1995, 56 (Suppl 1): 24-31.
24. Sarosiek J & Mccallum RW. Do Salivary Organic Components Play a Protective Role in Health and Disease of the Esophageal Mucosa? Digestion 1995; 56(Suppl 1): 32- 7
25. Shaw GY & Searl JP. Laryngeal Manifestations of Gastroesophageal Ref1ux before and after Treatment with Omeprazole. S Med J 1997; 90(11): 1115-22.
26. Cianci R, Fedeli G, Cammarota G, Galli J, Agostino S, Di Girolamo S, Maurig M, Gasbarrini G. Is the alkaline ref1ux a risk factor for laryngeal lesions? Am J Gastroenterol 2000; 95(9): 2398 (letter).
1 Otorhinolaryngology, Head and Neck Surgeon, Joint Professor, Santa Casa de Sao Paulo.
2 Otorhinolaryngology, Assistant Professor, Santa Casa de Sao Paulo.
Address correspondence to: Rua Prof. Artur Ramos, 183 cj.34 São Paulo SP 05414-000.