



Trabalho Clínico
Transposição transpterigoidea do retalho de fascia temporoparietal: um novo método para reconstrução de base de crânio após acessos endoscópicos expandidos
Transpterygoid Transposition of a Temporoparietal Fascia Flap: A New Method for Skull Base Reconstruction after Endoscopic Expanded Endonasal Approaches
Autores:
Felipe Sartor Guimarães Fortes (Ex-Médico Residente e preceptor do HCFMUSP. Fellowship Universidade de Pittsburgh) Pós-Graduando (doutorando) da FMUSP
Ricardo L. Carrau (MD) Professor of Otolaryngology and Head Neck Surgery - Universidade de Pittsburgh. Minimally Invasive endoNeurosurgery
Center, University of Pittsburgh Medical Center.
Carl H. Snyderman (MD ) Professor of Otolaryngology and Head Neck Surgery - Universidade de Pittsburgh. Minimally Invasive endoNeurosurgery Center, University of Pittsburgh Medical Center
Amin Kassam (MD) Chairman Neurosurgery Department - Universidade de Pittsburgh, Minimally Invasive endoNeurosurgery Center - University of Pittsburgh Medical Center.
Daniel Prevedello (MD) Neurosurgery Department - Universidade de Pittsburgh, Minimally Invasive endoNeurosurgery Center - University of Pittsburgh Medical Center.
Allan Vescan (MD) Otolaryngology and Head Neck Surgery - Universidade de Pittsburgh. Minimally Invasive endoNeurosurgery Center, University of Pittsburgh Medical Center.
Arlan Mintz (MD) Neurosurgery Department - Universidade de Pittsburgh, Minimally Invasive endoNeurosurgery Center - University of Pittsburgh Medical Center
Paul Gardner (MD) Neurosurgery Department - Universidade de Pittsburgh, Minimally Invasive endoNeurosurgery Center - University of Pittsburgh Medical Center
Carlos Diógenes Pinheiro Neto (Médico Otorrinolaringologista) Médico Preceptor do HC-FMUSP
Palavras-Chave
Transposição transpterigoidea, Retalho de fascia temporoparietal, Defeito dural amplo, Septectomia posterior prévia
Resumo
Introdução. Os acessos endoscópicos expandidos (EEAs) para ressecção de lesões da base do crânio podem resultar em defeitos amplos com risco significativo de fístula liquórica. A reconstrução deste defeitos, especialmente em pacientes com radioterapia prévia, são melhores reconstruídos com a utilização de retalhos vascularizados. O flap de Hadad-Bassagasteguy, um retalho nasoseptal vascularizado, é nosso método preferido para reconstrução. Porém, em pacientes submetidos a septectomia posterior ou em tumores que invadam a fossa pterigopalatina ou rostrum do esfenóide.
Métodos. Foi desenvolvida uma nova técnica para transposição do TPFF para cavidade nasal para reconstrução de defeitos de base de crânio. O retalho é confeccionado através de uma incisão hemicoronal e avançado para o defeito utilizando um túnel da região temporal para infratemporal associado a um acesso endoscópico transpterigoideo. O túnel de partes moles, da região temporal para infratemporal e então para a FPP é aberto através de dilatadores de traqueostomia percutâneo. Nós apresentamos descrição detalhada e análise retrospectiva de casos.
Resultados. Dois pacientes com fístulas liquóricas grandes e radioterapia prévia foram submetidos a reconstrução da base do crânio com o TPFF. A exposição obtida foi adequada para colocação do TPFF endonasal com cobertura completa do defeito. Ambos casos foram resolvidos sem morbidade adicional.
Conclusão. O retalho TPFF é um método confiável e versátil para reconstrução da base do crânio após EEAs. Para sua confecção é necessária incisão externa, desta forma, não é nosso método de escolha. É recomendado para casos de defeito dural amplo em pacientes com septecomia posterior prévia e radioterapia prévia.
Keywords
Transpterygoid transposition, temporoparietal fascia flap, large dural defects, previous posterior septectomy,
Abstract
Introduction. Endoscopic expanded endonasal approaches (EEAs) for the resection of lesions of the skull base can create large defects that present a significant risk of postoperative cerebrospinal fluid (CSF) leak. These defects, especially in patients with preoperative radiotherapy, are best reconstructed with vascularized tissue. The Hadad-Bassagasteguy flap, a pedicled nasoseptal flap, is our preferred method for reconstruction. This option is not available, however, in patients who underwent a previous posterior septectomy or in those with tumors that invade the pterygopalatine fossa (PPF) or sphenoid sinus rostrum.
Methods: We developed a new technique for the transposition of the TPFF into the nasal cavity to reconstruct skull base defects. The flap is harvested using hemicoronal incision and then advanced to the defect using a temporal-infratemporal tunnel and an endonasal transpterygoid approach. The soft tissue tunnel, extending from the temporal to the infratemporal and then to the PPF, is opened with percutaneous tracheostomy dilators. We present a detailed description and a retrospective review.
Results: Two patients with large CSF fistulas who had undergone preoperative radiotherapy were reconstructed transposing the TPFF. We obtained an adequate exposure for placing the flap endonasally with complete coverage of the skull base defect. Both CSF leaks were resolved without any morbidity.
Conclusion: The TPFF is a reliable and versatile method for the reconstruction of skull base after EEAs. Its harvesting requires an external incision; thus, it is not our preferred method of reconstruction. It is recommended for large dural defects in patients with previous posterior septectomy and radiation treatment.
Instituição: From the Departments of Otolaryngology and Head and Neck Surgery (F.S.G.F., R.L.C., C.H.S., A.K., A.V.) Neurological Surgery (F.S.G.F., R.L.C., C.H.S., A.K., D.P., A.V., A.M., P.G.), Minimally Invasive endoNeurosurgery Center, University of Pittsburgh Medical Center
Suporte Financeiro:
INTRODUCTION
During the past
decade, we expanded our indications for endonasal approaches as a result of a
better anatomic understanding of the skull base relationships from the
endoscopic perspective and the acquisition of significant experience.
Concomitant technical and technological advances such as the design of
specialized instrumentation and refinements of image-guided systems have
facilitated the exposure and resection of skull base and intradural lesions
using endoscopic expanded endonasal approaches (EEA).1-4 Reconstruction of the defects resulting from these
surgeries is challenging and is associated with a high rate of postoperative
cerebrospinal fluid (CSF) leaks. The reconstruction of skull base defects after
EEAs follows the same principles and goals as the reconstruction
after external
approaches: to recreate a barrier between the arachnoid apace and the sinonasal
tract to prevent a CSF leak and ascending infections.5-7 Reconstruction of small skull base defects has a
success rate of over 95%, independent of which tissue or technique (inlay or
overlay) is used for the repair. The use of vascularized tissue flaps does not
appear to be critical in this cases.8-12 EEAs for the resection of skull base lesions create
defects that compare, or in some cases surpass, the size of defects produced
with traditional craniofacial resections. Reconstruction of these defects is a
challenge that has limited the expansion of the endoscopic endonasal
approaches.6 A recent development, the Hadad-Bassagasteguy flap (HBF),7 is a pedicled flap of the nasal septum based on the
nasoseptal artery that has overcome this problem in the vast majority of cases.
We use this flap, when available, for all EEAs with the potential for a
postoperative CSF leak, thus resulting in a sharp decrease in our incidence of
postoperative CSF leaks.7 In patients
who have undergone previous EEAs that included a posterior septectomy or in
patients with tumors involving the posterior septum, rostrum of the sphenoid
sinus, or PPF, the blood supply to the HBF has been compromised; therefore,
this option is not available. To address this problem, we have developed two
additional pedicled flaps that can be used to reconstruct the EEA defect: the
inferior turbinate flap based on the inferior turbinate branch of the posterior
lateral nasal artery (a branch of the sphenopalatine artery [SPA]) and the
temporoparietal fascia flap (TPFF) transposed through a ranspterygoid tunnel.
Their use is based on the size of the defect and a history of preoperative
radiotherapy or chemoradiotherapy.
Multiple reports have addressed the use of the TPFF in a variety of other reconstructive settings.5,13-16 Similarly, the anatomy of the temporoparietal region has been described extensively.17-19 We will briefly review some of its most important aspects as it is relates to the preparation, transposition, and placement of the TPFF. We encounter five main soft tissue layers covering the temporoparietal region, from superficial to deep: skin, subcutaneous tissue, temporoparietal fascia (TPF), temporal fascia/pericranium, and the temporalis muscle. The skin and subcutaneous tissue contain numerous glands, capillaries, hair follicles, and fat. The TPF, or musculoaponeurotic layer, represents a continuation of the superficial musculoaponeurotic system and superiorly becomes the galea aponeurotica.
The subgaleal fascia, also called the "loose areolar layer," is attached to the frontal process of the zygoma and continues over the superior aspect of the zygomatic arch, above the external auditory meatus, and over the mastoid process. The temporalis fascia comprises superficial and deep layers that respectively attach to the superficial and deep aspect of the zygomatic arch and are continuous with its periosteum. Pericranium is a term that describes the loose areolar tissue and the periosteum of the skull. This latter layer is continuous with the periorbita and the deep temporalis fascia. The temporalis muscle originates at the temporal fossa and inserts on the coronoid process of the mandible.15,20 The TPF is a strong fascial layer that is connected to the overlying fibrous septae of the subcutaneous tissue above.15 The blood supply for the TPF comes from the superficial temporal artery (STA), which is one of the terminal branches of the external carotid artery (ICA). It courses through the retromandibular parotid gland, crosses the posterior root of the zygomatic process of the temporal bone, and becomes incorporated into the fascia at the level of the zygomatic arch.20 According to a previous study,18 the average diameter of the vessel is 2.73 mm over the zygomatic arch, and the mean distance between the STA and the tragus is 16.68 mm. In most patients, the STA divides into an anterior frontal and a posterior parietal branch at the level of the zygomatic arch (61%-88%); however, the bifurcation point can be superior to the arch (4%-26%) or inferior to the arch (7%-12%).18,19 One or two veins accompany the STA, which lies just deep to them.18 The frontal branch of the facial nerve courses just under the TPF after it crosses the superficial surface of the zygomatic arch.15,20 The TPF is 2 to 3 mm in thickness over the parietal region and extends in a fan-like manner from the preauricular region, comprising a surface area as large as 17 _ 14 cm.21 The deep temporal fascia consists of dense, white connective tissue layer that overlies the muscle and is continuous with the pericranium above the level of the superior temporal line.14 As previously mentioned, the distal insertion of the temporalis muscle is the tip and medial surface of coronoid process and the anterior border of ramus of mandible. The temporalis muscle passes deep to the zygomatic arch and, along with the deep temporal fat pad, forms the lateral boundary of the infratemporal fossa (ITF).17 This communicates the temporal fossa with the ITF, which is an irregularly shaped space posterior to the maxilla and limited medially by the lateral pterygoid plate.4,17 The lateral and medial pterygoid muscles are located in the ITF medial to the temporalis muscle. The aim of this report is to describe a new technique for the reconstruction of skull base defects without the need for maxillofacial osteotomies. This technique involves the transposition of the TPFF into the nasal cavity through a temporal-infratemporal soft tissue tunnel and a transpterygoid window.
MATERIALS AND METHODS
We retrospectively reviewed the demographic, clinical, and surgical and outcome data of two patients who underwent reconstruction of the skull base using the TPFF. These two patients presented a communication between the subarachnoid space and the sinonasal tract and exposure of at least one ICA.
Surgical Technique
It should be noted that we have ample experience with the use of the TPFF and that we designed and tested the feasibility of the transpterygoid technique in cadaveric specimens before trying it in a patient. Harvesting and transposition of the TPFF to the nasal cavity is carried after the final defect size and site can be determined. It may be harvested from either side of the scalp, but we prefer the side that is ipsilateral to the defect or EEA. Previous incisions over the area of the flap may alter the laterality of the harvesting. If not performed previously as a part of the EEA, we first complete an anterior and posterior ethmoidectomy and a large maxillary antrostomy. Then, the SPA and posterior nasal artery are identified and clipped at the level of the sphenopalatine foramen. A retrograde dissection of the SPA into the PPF, using Kerrison's rongeurs, also serves to remove the posterior of the lateral wall of the maxillary sinus is removed also to open a wide communication with the ITF. The descending palatine artery is identified in its inferior vertical trajectory from the IMA and is dissected from its canal. This allows the inferior and lateral displacement of the contents of the upper PPF, therefore exposing
the pterygoid plates. The pterygopalatine ganglion may be preserved, but the vidian nerve has to be divided to allow the displacement
of the ganglion. Then, the anterior aspect of the pterygoid plates is reduced with a high-speed drill to enlarge the space for the transposition of the TPFF. It should be noted that at this point, we should have full access into the ITF and that the IMA should be completely mobile at the pterygomaxillary fissure. We then harvest a TPFF from the ipsilateral side using a conventional technique.
A hemicoronal incision is carried down to the level of the hair follicles. Care must be taken to avoid injuring the pedicle during the hemicoronal incision (Fig. 1). Dissection of the TPF from the subcutaneous tissue effectively elevates the flap (Fig. 2). Once enough surface area is exposed, the fascia is incised at its lateral margins and is elevated from the cranium and deep temporal fascia down to its pedicle. The superficial layer of the deep temporal fascia is incised vertically, and the fascia is separated from the muscle following this plane of dissection inferiorly to elevate the periosteum from the surface of the zygomatic arch. This creates a wide tunnel beneath the superficial layer of the deep temporalis fascia that will accept the passage of the pedicle without compression. To help the transposition of the TPFF, a lateral canthotomy incision is used to expose and separate the temporalis muscle from the lateral orbital wall and from the pterygomaxillary fissure (Fig. 4). This creates a tunnel that communicates the temporal, the infratemporal fossa, and the transpterygoid approach. This soft tissue tunnel is sequentially dilated by passing a guidewire into the nose under endonasal endoscopic visualization and then advancing percutaneous tracheotomy dilators over the wire (Fig. 3). After an adequate tunnel is obtained, the dilators are removed, and the flap is tied to the external end of the guidewire. As the nasal end of the guidewire is pulled out through the nostril, it pulls the flap through the tunnel into the nasal cavity. The mobilization of the flap through the tunnel is assisted with external manipulation. It is important to avoid a rotation of the flap because this may compromise its blood supply. The external incisions are closed with a running nylon 4-0 stitch after insertion of a suction drain. The reconstruction of the skull base begins with the placement of an inlay graft of a collagen matrix. The edges of the defect are refreshed, and the TPFF is placed over the defect (Fig. 5). Fibrin glue or other sealant may be used after the flap is in position. The flap is then covered with Gelfoam, and a sponge packing is placed to stabilize the flap. We remove the packing 3 to 5 days later.
RESULTS
We have used the TPFF in two patients with large skull base defects that resulted in a CSF leak and an exposure of at least one ICA. One patient presented with a clivus chordoma recurrent after surgery and treatment with proton beam. The tumor compressed the right optic nerve and ICA. An EEA was performed for tumor debulking and optic nerve
exposure of the right ICA (Fig. 9). The second patient also had a history of a recurrent clival chordoma that was treated with multiple surgical procedures, two courses of gammaknife therapy, and proton beam therapy. This patient presented with osteoradionecrosis of the clivus and remaining walls of the sphenoid sinus months after proton beam therapy, which was delivered after an uneventful EEA. This resulted in a large area of radionecrosis, exposure of both vertical and parasellar ICAs, and a CSF fistula associated with meningitis and subdural empyema (Fig. 10). In both cases, the TPFF healed uneventfully, and we encountered no complication from the access surgery or the harvesting. We observed mucosalization of the flaps after 3 to 4 weeks.
DISCUSSION
The reconstructive goals after skull base surgery, whether the defect is created by endoscopic or by external approaches, include the separation of the cranial cavity from the sinonasal tract, the obliteration of dead space, function.5,20 Some critical factors that affect the reconstructive planning include the size and site of the defect, size and geometry of the remaining dural and bone margins, a history of previous intranasal surgery that involved wide sphenoidotomies or a posterior septectomy, history of preoperative radiotherapy treatment, general status of the patient, and the need for postoperative radiotherapy.6,8 Multiple authors have reported various free grafting techniques for the closure of CSF leaks through an endoscopic endonasal approach.9-11 EEAs for the resection of skull base lesions, however, create large defects that communicate the subarachnoid space with the sinonasal tract and are associated with a high incidence of postoperative CSF leaks. We have refined our free grafting technique on the basis of the lessons learned during the exploration of our own series of postoperative CSF leaks. Among other modifications, we developed a multilayer technique using a collagen matrix as a subdural inlay graft and an acellular dermis as an onlay graft followed by an onlay abdominal fat graft to be supported by a 12 F Foley catheter for approximately 5 days.6,22 This technique reduced our initial incidence of postoperative CSF leaks by half, but the incidence remained higher than desirable. This prompted us to develop more reliable and safer reconstructive techniques that could be applied without the need for a craniotomy or maxillofacial osteotomies.6,7 Reconstructive and functional outcomes after skull base surgery are dependent on rapid and uninterrupted healing of the surgical site; therefore, reconstruction of the skull base with vascularized flaps appears advantageous.5 Vascularized flaps promote adequate and a rapid healing that decrease the possibility of CSF leaks, pneumocephalus, and ascending meningitis. These benefits are critical for the reconstruction of very large defects and for defects in patients who have received preoperative radiotherapy or chemoradiotherapy. In addition, the complete and accelerated healing facilitated by the vascular flaps may allow for the earlier and safer delivery of postoperative radiation therapy.5,15,16,20 Our incidence of postoperative CSF leaks remained high until we adopted the HBF. This flap reduced the postoperative CSF rate incidence to a level that compares with that of traditional approaches (approximately 5%).7 The HBF is a pedicled flap that includes the nasal septum mucoperiosteum and mucoperichondrium. It is supplied by the nasoseptal artery, a branch of the posterior septal artery, which is one of the terminal branches of the internal maxillary artery.7 Because of its vascular pedicle, the HBF is versatile, reliable, and has a great arc of rotation that can cover a large surface area. It is our preferred method for reconstruction of the skull base defects associated with CSF leaks or exposure of the ICA after EEAs.7 Its main disadvantage is that its use must be anticipated before a posterior septectomy is performed because this would interrupt its vascular supply. In these cases, most frequently after a prior EEA, we can choose one of the two other pedicled flaps: the posterior pedicle inferior turbinate flap (PPITF) and the TPFF. The PPITF is based on the inferior turbinate branch of the SPA and is more suitable for patients with limited defects. Bilateral PPITFs may be harvested, but the surface area reach is still limited by the dimensions of the turbinate; therefore, for large defects, especially in patients who have undergone preoperative radiotherapy, the TPFF is a superior option.5,16,20 The TPFF is a pedicled flap based on the anterior branch of the STA with remarkable intrinsic versatility. It allows for the reconstruction of a variety of defects including intraoral defects, oronasal and nasocutaneous fistulas, and skull base defects after a traditional craniofacial resection, among others.13-16,20 Others have used it as a free microvascular flap. The TPFF has a predictable vascular anatomy, good arc of rotation, long vascular pedicle length, and a rich vascularity that allows it to survive and heal even in unfavorable conditions, such as those existing in patients who received prior radiotherapy.15,20,21 Soft tissue and bone fibrosis, poor blood supply, and sometimes necrosis is present in these latter patients.5,16,20 The TPFF is thin and pliable, which eases its adaptation to a variety of clinical settings, irregular surfaces, and complex geometries. It can cover a considerable surface area that can be as large as 17 _ 14 cm, making it an option for moderate to large defects of the anterior, middle, clival, and parasellar skull base. In addition, it may be folded to provide multilayer coverage.21 Mucosalization of the surface of the flap, inside the nasal cavity, occurs quickly.20 Some important aspects must be remembered to avoid complications: the surgeon must not cross an imaginary line between the tragus and the lateral eyebrow or dissect anterior to the hairline to preserve the frontal branch of the facial nerve that runs deep to the TPFF; also, careful dissection between the TPFF and the subcutaneous tissue will help to minimize the risk of alopecia. 13-15 These complications were not observed in either of our two cases. Others have described an endoscopic harvesting of the TPFF to minimize the risk of alopecia and visible scar, especially in bald males.23 Our technique allows the TPFF to repair defects of the skull base by transposing the flap from the temporal to the infratemporal fossa and then advancing it into the nasal cavity through the PPF (transpterygoid tunnel). The EEA to the infratemporal fossa can be accomplished with techniques that have been previously described in the literature.4 Although we have used the TPFF for skull base reconstruction after EEA only in two cases only, our previous experience using this flap for traditional craniofacial resections has proven that it is safe and reliable. Disadvantages of this technique include the need for an external approach and a surgical scar, risk of alopecia, damage to the frontal branch of the facial nerve, and the need for an associated EEA access to the to the infratemporal fossa.
CONCLUSION
The TPFF is a reliable reconstructive method that may be used to repair extensive defects of the anterior, middle, clival, and parasellar skull base resulting from an EEA, especially in patients who have a history of a posterior septectomy or previous radiotherapy.
Figura 1.
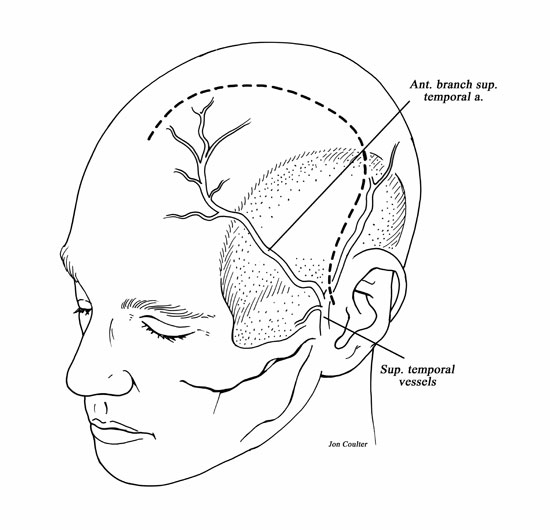
Drawing of the left hemicoronal incision.
Figura 2.
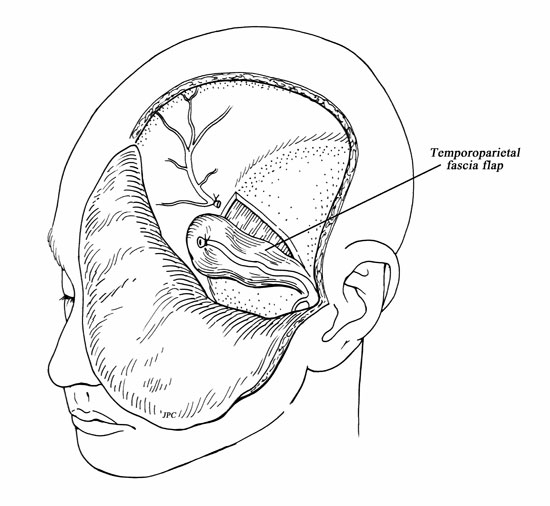
Drawing of the temporoparietal fascia flap.
Figure 3.
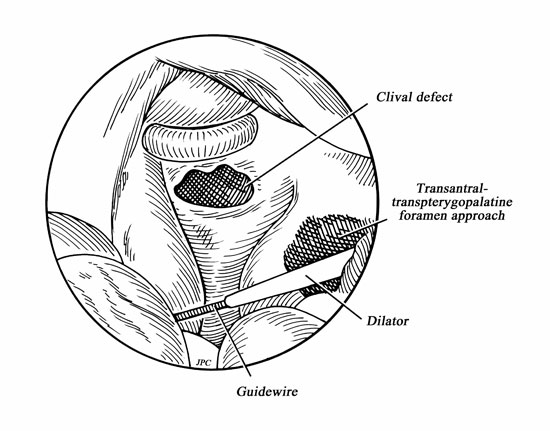
Drawing of guidewire and dilator being advanced into nasal cavity.
Figure 4.
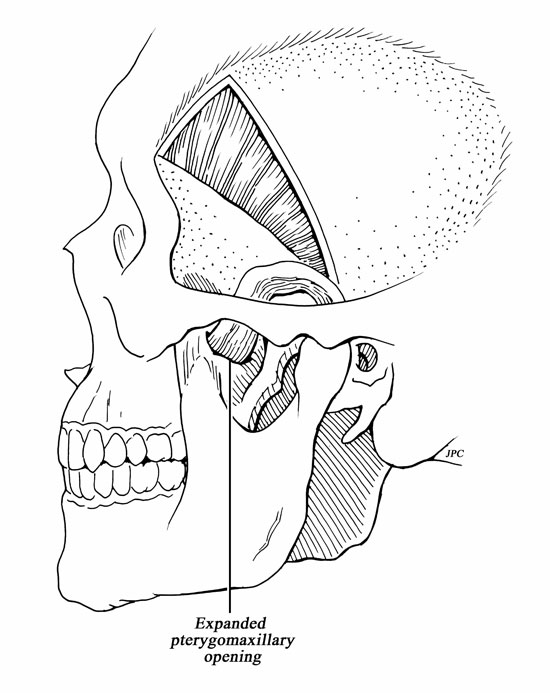
Drawing of the transposition of the TPFF and the expanded maxillary opening.
Fgure 5.
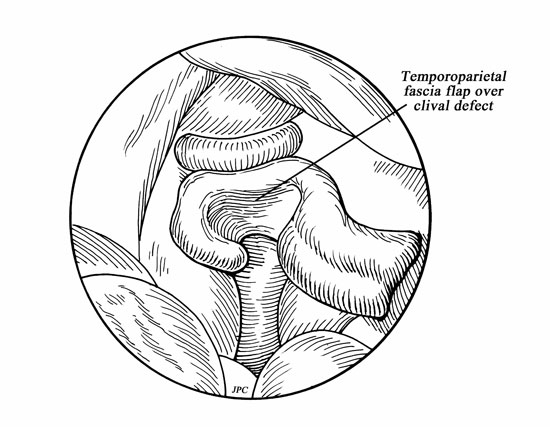
Drawing of temporoparietal fascial flap covering cerebrospinal fluid fistula site.