Trabalho Experimental
Anatomia endoscópica da fossa pterigopalatina:
desenvolvimento de modelo anatômico
Endoscopic Anatomy of the Pterygopalatine Fossa:
Development of an anatomical model
Autores:
Felipe Sartor Guimarães Fortes (Ex-residente e médico preceptor do HCFMUSP.) Pós-Graduando (doutorando) da Disciplina de Otorrinolaringologia do HCFMUSP
Luiz Ubirajara Sennes (Professor Livre-Docente ) Professor Assosciado da Disciplina de Otorrinolaringologia da FMUSP
Ricardo Carrau (MD) Professor da Universidade de Pittsburgh - Otolarynoglogy and Head Neck Surgery
Rubens V. de Brito Neto (Professor Livre-Docente) Médico Assistente do HCFMUSP. Grupo de Otologia e cirurgia de base de crânio.
Guilherme C. Ribas (Professor Livre-Docente) Professor do Departamento de Cirurgia da FMUSP - Disciplina de Topografia Estrrutural Humana
Alexandre Yasuda (Doutor em Neurocirurgia pela FMUSP) Departamento de Cirurgia da FMUSP - Disciplina de Topografia Estrrutural Humana
Aldo J. Rodrigues Jr. (Professor Livre-Docente) Professor Titular da Disciplina de Topografia estrutural Humana FMUSP
Carl H. Snyderman (MD ) Professor da Universidade de Pittsburgh - Otolarynoglogy and Head Neck Surgery
Amin Kassam (MD) Chairman Neurosurgery Department - Universidade de Pittsburgh
Palavras-Chave
Anatomia, Fossa pterigopalatina, Base de crânio, Cirurgia endoscópica, Artéria carótida interna, Artéria Maxilar
Resumo
Introdução. A fossa pterigopalatina (FPP) é um espaço estreito localizado entre a parede posterior do maxilar e o processo pterigóide. O acesso cirúrgico a esta região é difícil devido a sua localização protegida e complexa anatomia neurovascular. O acesso endoscópico permite melhor visualização com menor morbidade em relação aos acessos externos. Nosso objetivo é desenvolver um modelo anatômico através da injeção intravascular de silicone corado para demonstrar a anatomia endoscópica.
Métodos. 6 FPP foram preparadas para dissecção utilizando duas técnicas diferentes de injeção. Um acesso endoscópico endonasal através do meato médio com remoção do parade posterior do seio maxilar foi utilizado para estudar a FPP e suas relações com a base do crânio.
Rersultados. A injeção de silicone corado na artéria carótida comum permitiu desenvolvimento do melhor modelo anatômico. A artéria maxilar interna foi identificada através de dissecção retrógrada da artéria esfenopalatina. As estruturas neurais localizam-se profundamente as estruturas vasculares. Reparos anatômicos importante são o canal do vidiano para a artéria carótida interna (porção petrosa) e o forame redondo (V2) para o cavum de Meckel na fossa média.
Conclusão. Nosso modelo anatômico permite o estudo endoscópico da FPP e a simulação de técnicas cirúrgicas. O acesso endoscópico endonasal permite exposição adequada de todas estruturas anatômicas da FPP, que podem ser utilizadas para o controle de estruturas mais profundas na base do crânio.
Keywords
Anatomy, Pterygopalatine Fossa, Skull base, Endoscopic surgery, Internal carotid artery, Maxillary artery
Abstract
Introduction. The pterygopalatine fossa (PPF) is a narrow space located between the posterior antrum wall and the pterygoid plates. Surgical access to the PPF is difficult due to its protected position and its complex neurovascular anatomy. Endonasal approaches provide a better visualization of this area with less morbidity than external approaches. Our aim was to develop an anatomical model using cadaveric specimens injected with intravascular colored silicone to demonstrate the endoscopic anatomy.
Methods. We dissected six PPF prepared with intravascular injection of colored material using two different injection techniques. An endoscopic endonasal approach, including a wide nasoantral window and removal of the posterior antrum wall, provided access to the PPF.
Results. We produced our best anatomical model injecting colored silicone via the common carotid artery. We found that a retrograde dissection of the sphenopalatine artery helped to identify the internal maxillary artery (IMA) and its branches. Neural structures were identified deeper to the vascular elements. Notable anatomical landmarks for the endoscopic surgeon are the vidian nerve and its canal that leads to the petrous portion of the internal carotid artery (ICA), and the foramen rotundum, and V2 that leads to Meckel's cave in the middle cranial fossa.
Conclusion. Our anatomical model provides the means to learn the endoscopic anatomy of the PPF and for the simulation of surgical techniques. An endoscopic endonasal approach provides adequate exposure to all anatomical structures within the PPF. These structures may be used as landmarks to identify and control deeper neurovascular structures.
Instituição: Disciplina de Otorrinolaringologia HCFMUSP, Minimally Invasive endoNeurosurgery Center - University of Pittsburgh Medical Center, Human Topographic Anatomy Division - Department of Surgery FMUSP
Suporte Financeiro: FAPESP - Fundação de Amparo a Pesquisa do Estado de São Paulo
Introduction.
Endoscopic approaches are fundamental for the surgical treatment of infectious and inflammatory diseases of the sinonasal tract. Recent developments including new endoscopic techniques, powered instrumentation and imaging guided systems, as well as the increasing familiarity of the endoscopic surgeon with the skull base anatomy have expanded the indications for endoscopic endonasal approaches. Other pathologies involving the paranasal sinuses and adjacent areas are now approached and treated using this techniques.1-4
Access to the PPF is a surgical challenge, due to its location deep in the mid-third of the face and its complex array of vascular and neural structures. It comprises the internal maxillary artery (IMA) and its branches, the maxillary nerve (V2) and its branches, the pterygopalatine ganglion (PPG), and the vidian nerve (VN). 5 All these structures are contained in a narrow space shaped as an inverted pyramid, located between the posterior wall of the maxillary sinus (PWA) and the pterygoid plates. Its boundaries are the pterygoid plates (PP) posteriorly, the ascending process of the palatine bone anteromedially and the PWA anterolaterally, the pterygomaxillary fissure (which connects the infratemporal fossa, ITF, to the PPF) laterally, the middle cranial fossa and greater sphenoid wing superiorly, and the pyramidal process of the palatine bone inferiorly.5, 6 An important aspect of the PPF is its topographical relation and connections with the orbit and cranial cavity. Pre-existing pathways allow the direct dissemination of tumors and inflammatory diseases into the cranial base and middle cranial fossa.7, 8
Endonasal endoscopic approaches (EEAs) avoid external incisions, maxillofacial osteotomies and craniotomies; thus, reducing the potential morbidity and shortening the recovery period, while providing excellent illumination and image magnification of the surgical field. These characteristics are advantageous especially when dealing with areas that are difficult to access such as the pterygopalatine fossa (PPF). 3,4 The endoscopic endonasal approach to the PPF and its boundaries has been described by many authors, 1,9,10,11 and is currently used to approach various neoplastic and inflammatory diseases.3, 4, 12 In addition, it may be used to treat lesions in the lateral recess of the sphenoid sinus, which represents an extended pneumatization of the sphenoid bone laterally just posterior to the PPF.13
A thorough anatomical knowledge of the PPF is mandatory before attempting these approaches even for surgeons with superior endoscopic skills. The objective of this study was to develop an anatomical model that could illustrate the endoscopic anatomy of the PPF, familiarize the endoscopic surgeon with its complex anatomy, and allow the simulation of different surgeries pertinent to this area.
Materials and Methods:
This study was developed at the Human Topographic Anatomy Lab of the University of São Paulo Medical School. Our local institutional research committee approved the project.
Six pterygopalatine fossae in three
fresh cadaveric heads were prepared for dissection after the intravascular injection
of colored liquid silicone, using a previously described technique.14
The specimens were stored in a 75% alcohol solution, and prepared for
dissection. Both the internal carotid artery (ICA) and the internal jugular
vein (IJV) were identified and prepared for bilateral injection in the cervical
region. In two specimens, the first segment of the IMA was dissected and
isolated for the injection of silicone. In one specimen the common carotid
artery (CCA) was dissected and isolated for the injection, without the direct
injection in the IMA. All vessels were
cannulated using a catheter of a gauge that would fit the lumen snugly. Then,
the cannulated vessels and catheters were tied with a # 0 cotton suture to avoid
spillage of the dye. The vessels were flushed repeatedly with water using a 60
cc syringe until we obtained uninterrupted flow from its contralateral
counterpart. For instance, we flushed the right internal carotid artery, until
water would come out of the left internal carotid artery and we could not
longer see any blood clots inside the vessels. This step is critical, as
inadequate flushing or clots would prevent the dye from filling up the vascular
system, especially small arterial branches. Both the carotid and the vertebral
arteries were injected through their cervical segments, and the internal
jugular vein was injected right after the arterial injections. We used red dye
for arteries and blue dye for the venous system, using the following
formulation:
A) Arteries: two parts polymethyl silaxane (thinner) to one part silicone.
B) Veins: one part polymethyl silaxane (thinner) to one part silicone.
The catalyst (dilaurate calcium carbonate) was added before injecting the solution in the vessel, in a ratio of 10cc for each 300cc of solution. The dye (water soluble pigments) was added right before the infusion also. During the endoscopic dissection of the PPF, photographs were taken with an SLR digital Nikon D70® of 6.5 mega pixels coupled to the endoscope. Digital images were then stored in a laptop computer using the Windows Picture Viewer.
Dissection technique
We identified the anatomical landmarks of the middle and superior meati. Then, we performed an anterior and posterior ethmoidectomy and a wide nasoantral window, identifying and enlarging the maxillary ostium anteriorly and posteriorly. The mucosa of the posterior and the superior walls was removed. A transethmoidal sphenoidotomy was performed and the mucosa and bone over the ICA, optic nerve (ON), pituitary (P), and cavernous sinus (CVS) were removed to reveal the topographic relations of the region (Figure 1a). We clipped the sphenopalatine artery (SPA) and posterior nasal artery (PNA) after identifying and removing the crista ethmoidalis (Figure 1b).
Our surgical exposure continued with the removal of the cephalic half of the posterior wall of antrum and the ascending process of the palatine bone using Kerrison's rongeurs. Opening of the periosteum and removal of the fat from the PPF revealed the proximal SPA and PNA, which then were followed retrogradely to the IMA (Figure 2 a-b). Inferior displacement of the IMA and its branches allowed the visualization of the pterygopalatine ganglion (PPG). At this point it was possible to see the PPG, branches from V2, vidian nerve and descending palatine nerve (DPN) (Figure 3 a-b). An inferior turbinectomy, removal of the adjacent lateral wall of the nose and the inferior half of the PWA allowed the visualization of the entire height of the PPF and its contents. (Figure 4)
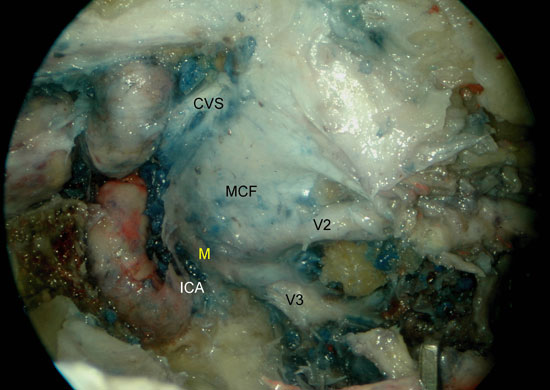
Retrograde dissection of efferent fibers from the PPG and displacement of the other structures of the PPF facilitated the identification of the vidian nerve and its canal (Figure 5 a-b). At this point we drilled the sphenoid sinus floor preserving the vidian canal to study the relation of the vidian nerve with the ICA (fig. 6a). We identified the infraorbital nerve (ION) (Figure 3b) and the trigeminal second and third divisions (V2 and V3) as they entered the foramen rotundum and foramen ovale, respectively (Figure 6 a-b).
Results.
We were able to expose and dissect the PPF endoscopically in all specimens. The identification of all branches of the IMA, however, was difficult, especially in those specimens where the dye was injected directly into the IMA. In these specimens, the highlighting of the IMA and its branches was inadequate for good documentation. Injection of the common carotid yielded a superior demonstration of the vessels. The CVS and ICA were very well colored in all the cases and the consistency and density of the injected material was adequate, making it possible to continue the dissection, which remained with good quality for documentation, even in the presence of leakage from small vessels.
In the cadaveric dissections, it was possible to preserve the middle turbinate. The removal of the PWA, which was thin in all cases, was accomplished using Kerrison's rongeurs. These instruments were adequate to remove the palatine bone even when this bone is significantly thicker than the PWA. The first structure we encountered after removal of the PWA was the periosteum of the posterior aspect of the maxilla, covering the fat of the PPF. Upon the initial opening of the periosteum it was somewhat difficult to identify the vessels due to the surrounding fat and the tortuous and variable course of the IMA. We found that early identification and proximal dissection of the SPA was very useful in finding the IMA. We did not find any significant veins in our dissections.
The anterior compartment of the PPF was occupied by fat and vascular structures, while the neural elements lied posteriorly. In all specimens the first neural element to be identified was the ION as it runs in the superior wall of the maxillary sinus. Removal of the antral mucosa facilitated its visualization. We followed the ION retrograde and identified the more proximal V2 as it enters the inferior orbital fissure. V2 arises from the trigeminal ganglion at Meckel's cave (middle cranial fossa) to run anteriorly and then exit the skull base through the foramen rotundum. In the PPF, V2 gives a branch to the PPG, before continuing as the ION. The ION continues through the inferior orbital fissure, which is continuous with the pterygomaxillary fissure. The latter marks the limit between the PPF and ITF; therefore, the ION may be used as a proxy landmark to indicate the boundary of the ITF.
We identified the greater palatine nerve (GPN), just medial to the descending palatine artery (DPA) and following an inferior vertical trajectory from the PPG. The PPG has a triangular shape and lies posterior to the SPA. We could identify all three principal branches: the descending palatine nerve inferiorly; a branch of V2 superolaterally; and the vidian nerve superomedially.
The dissection continued identifying the vidian nerve, proximal to the PPG, as it runs in its canal along the sphenoid sinus floor in a posterolaterally direction. In all cases, the vidian nerve met the second genu of the ICA (between the horizontal and vertical segments). After identification of the ICA anterior genu, we removed the bone covering the final segment of the horizontal petrous ICA and identified the foramen ovale and V3, both of which are positioned superior, lateral and anterior to the ICA. Following the course of V2 proximally we reached the dura mater of the middle cranial fossa. V2 along with V3 mark the Gasserian ganglion just superior to the horizontal segment of the ICA.
Discussion.
A variety of infectious, neural, vascular, and neoplastic diseases may occur in the PPF, lateral sphenoid sinus, and retrobulbar orbit.3, 13 A surgical approach to or through the PPF may be necessary for drainage, histological sampling, or definitive surgical treatment.4, 15
Surgical access to this region, however, is a surgical challenge. A complex array of vascular and neural structures characterizes this narrow space. Its surgical anatomy is further complicated by anatomic variations and its protected position. Identification of these structures may be difficult, especially when bleeding or space occupying lesions obscures them. Traditional approaches to the PPF usually involve an anterior transmaxillary technique.1 The transoral-transantral approach requires the transgression of the anterior and posterior walls of the maxillary sinus. It is associated with complications, such as facial edema and pain, oroantral fistula, chronic maxillary sinusitis, vascular injury, injury the infra orbital nerve (ION), and dental injury.1, 9 Lateral approaches to expose and resect malignant or invasive tumors have been advocated also. 10, 16, 17 Endoscopic approaches to the PPF, however, provide superior visualization, facilitating the identification of the IMA and its branches and the identification of important neural structures.1, 2 In addition, they provide access to the adjacent regions, which are frequently affected due to their intimate relationship with the PPF structures.3, 10, 11 These areas include the nasal cavity, orbit, ITF, middle cranial fossa and nasopharynx. Access to the latter three areas requires control of the ICA and/or CVS, which are achieved by the EEA also (Figure 1a; 6a-b). 9, 11
Intravascular injection of the vessels with colored silicone was successful in enhancing the different segments of the ICA and the medial and lateral CVS. Identification of the IMA and its branches, however, required injecting material via the CCA and not directly in the IMA. This is probably due to the difficulty in flushing blood clots when using a direct injection into the IMA.
The IMA is a terminal branch of the external carotid artery, usually arising behind the neck of the mandible (mandibular segment), passing anteriorly and traversing the ITF over the lateral pterygoid muscle (pterygoid segment) to enter the PPF through the pterygomaxillary fissure.7, 8 The pterygopalatine segment, or third portion of the IMA, lies anterior to the PPG, giving origin to several branches. Their number and origin vary considerably. They may arise directly from the IMA or from other branches. A common pattern of branching from proximal to distal include the posterosuperior alveolar artery (PSAA) and infraorbital artery (IOA), which are located at the posterior wall of the maxilla; then, in the PPF proper, the IMA gives branches to the palate (DPA), nasal cavity (SPA and PNA), pterygoid canal, and may give origin to the pharyngeal artery (PA) that runs to the nasopharynx through the palatovaginal canal.7, 8
In our six specimens the IMA bifurcated into the PNA and SPA proximal to the sphenopalatine foramen (Figure 1a); and, the crista ethmoidalis was a consistent landmark just anterior to the foramen and arteries (Figure 2b). It should be remembered that the origin and relationships of these vessels are variable, so that the SPA and PNA may originate from the IMA and exit the PPF through one, two or more foramina, and the bifurcation may occur proximal or distal to the sphenopalatine foramen. 7, 18 As the SPA and the PNA are branches of the IMA, a proximal dissection of either vessel will lead to the IMA (Figure 3 a-b).
The PPF is often devoid of identifiable veins, as observed in our specimens. A large posterior venula, however, may run vertically in the mucoperiosteum. 8
After removing the PPF fat and isolating the vascular structures, we were able to identify the more posterior neural elements (Figure 4). The volume of fat within the fossa varies; and those with less fat are easier to dissect. 8 The PPG is located just posterior to the SPA before it exits the sphenopalatine foramen and has three principal branches: the vidian nerve, a branch from V2, and the greater palatine nerve (Figure 3a). 9 It innervates the mucus and lacrimal glands, and provides sensory afferents to the palate through the palatine nerves.5 Anesthesia or hypoesthesia of the palate has been described as complication from SPA ligation or PPF surgeries; thus, the nerve should be identified and preserved whenever possible.19 The vidian nerve (or nerve of the pterygopalatine canal) is formed by parasympathetic fibers from the facial nerve through the greater petrosal nerve, and sympathetic fibers from the internal carotid plexus through the deep petrosal nerve.5 The relevance of this nerve for endoscopic approaches to the lateral and posterior skull base is that it serves as a landmark to identify the anterior genu of the ICA (Figure 6a). This was described by previous authors9, 11 and observed during our dissections. We identified this nerve by following the PPG, but a more obvious endoscopic landmark is the junction of the medial pterygoid plate with the sphenoid floor. The vidian nerve is located just lateral to this point.11
The ION crosses the superior wall of the maxillary along with its artery, after exiting the PPF via the inferior orbital fissure.1, 5 This nerve is an important anatomical landmark, as it marks the boundary between the PPF from the ITF and leads to V2 (Figure 3b). The V2 is a branch of the trigeminal nerve, and before leaving the cranial cavity via the foramen rotundum and has an intracranial course along the dura mater of the middle cranial fossa. This portion of the V2 is superolateral to the ICA; thus, constituting an important landmark to the endoscopic suprapetrous approach (Figure 6b). 11 V3 is a sensory and motor nerve located lateral and inferior to V2 as it leaves the skull base toward the ITF via the foramen ovale superior to the horizontal ICA (Figure. 6b). 5, 9 A pyramidal shaped bone separates the vidian nerve and V2. Its base is at the anterior face of the pterygoid plates and, its apex, located posteriorly, marks the ICA.
The greater palatine nerve has a vertical and caudal trajectory from the PPG to supply afferents fibers to the palate (Figure 3a).19 We can identify the nerve medial to the DPA and close to the lateral nasal wall in a plane inferior to the SPA. According to Mellema and Tami19, this distance may vary from 3 to 5 mm. Careful instrumentation is important while working in the lateral nasal wall to avoid injury to this nerve.
Conclusion.
The injection of colored silicone in the common carotid artery facilitates the identification and dissection of the IMA and its branches for a cadaveric demonstration of the endoscopic approach to the PPF. This anatomical model may help the endoscopic surgeon to learn the anatomy of the PPF and to simulate different surgical techniques to and through this area. In our study, all the PPF structures were well exposed via the endoscopic mid-transantral approach. The visualization of the IMA and its branches in the PPF is possible through this approach since they are contained in the mid third of the PWA8. However, visualization of the IMA segment entering the PPF from the pterygoid muscles required the removal of the inferior aspect of the lateral wall of the nose. The neural structures of the PPF are located deep to the IMA and its branches, therefore, a dissection that remains superficial to these vessels avoid injury to the neural elements.
The PPF offers a direct route to the lateral skull to the endoscopic surgeon. Awareness of the anatomical relationships of the vidian nerve and V2 are critical for the identification and control of the ICA and the middle cranial fossa.
References.
Figura 1A
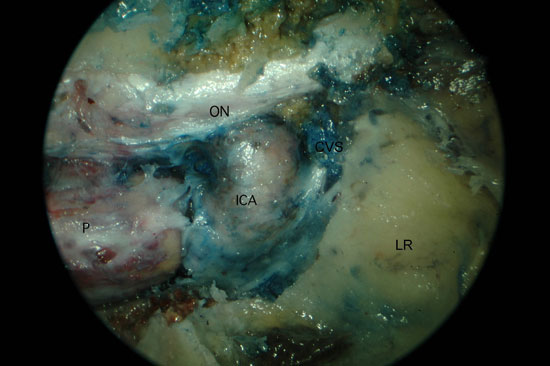
Figure 1. Left PPF approach. A) Sphenoid sinus with removal of the posterior bone wall. B) Large maxillary sinus opening with identification of the SPA. P, Pituitary; ON, optic nerve; ICA, internal carotid artery; CVS, cavernous sinus; LR, lateral recess of sphenoid; PAW, posterior antrum wall; CE, crista ethmoidalis; LP, lamina papyracea.
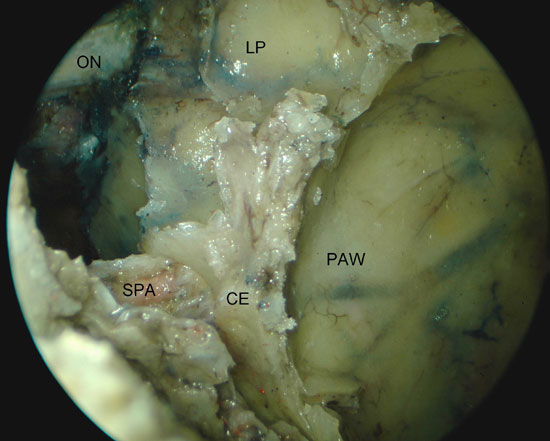
Figura 2A
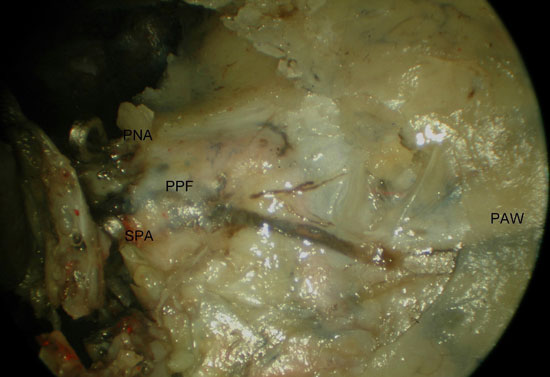
Figure 2. Access to the left PPF with identification of the IMA (left approach). A) the maxillary posterior wall has been partially removed exposing the PPF contents within fat and periosteum. B) after opening the PPF fascia and partial removal of the fat, the SPA was followed to the IMA. PPF, pterygopalatine fossa; SPA, sphenopalatine artery; PNA, posterior nasal artery; PAW, posterior antrum wall; MT, middle turbinate; OA, orbital apex; PN, greater palatine nerve; DPA, descending palatine artery; IMA, internal maxillary artery.
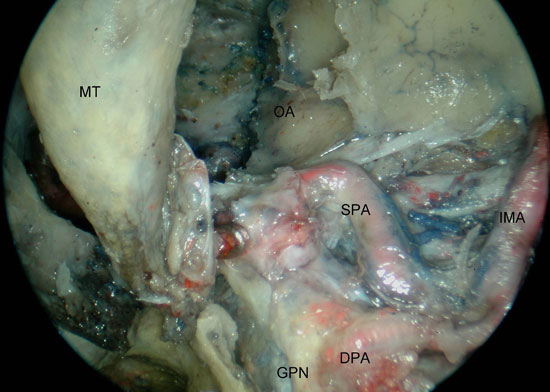
Figura 3A
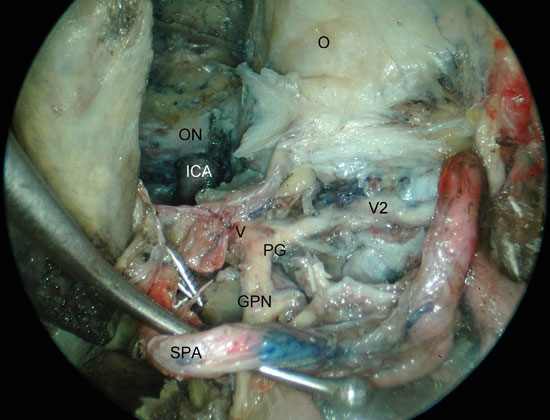
Figure 3. Left PPF approach. A) identification of the PPG after inferior traction of the SPA. B) visualization of the relation between V2, infraorbital nerve and the limit to the infratemporal fossa. ICA, internal carotid artery; O, orbit; ON, optic nerve; V2, maxillary nerve; PG, pterygopalatine ganglion; GPN, greater palatine nerve; V, vidian nerve; IOW, infra-orbital wall; ION, infra-orbital nerve; IMA; internal maxillary artery; ITF, infratemporal fossa.
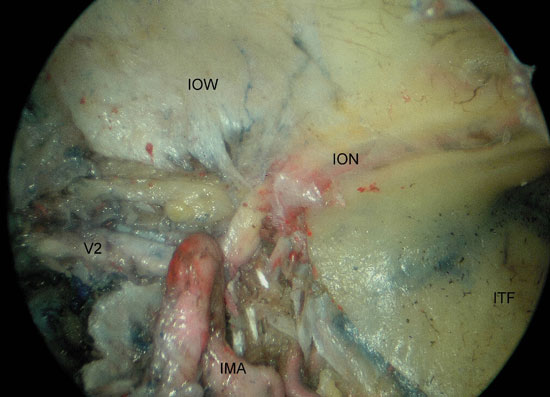
Figura 4
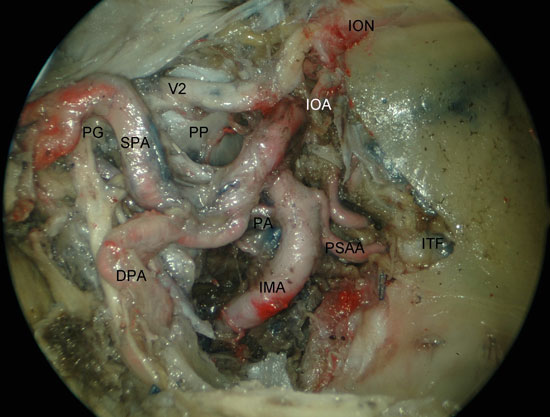
Figure 4. Visualization of the left PPF after medial maxillectomy. PG; pterygopalatine ganglion; DPA, descending palatine artery; SPA, shenopalatine artery; PA, pharyngeal artery; IMA, internal maxillary artery; BA, buccal artery; IOA, infraorbital artery; V2, maxillary nerve; ION, infraorbital nerve; ITF, infratemporal fossa; PP, pterygoid process.
Figura 5
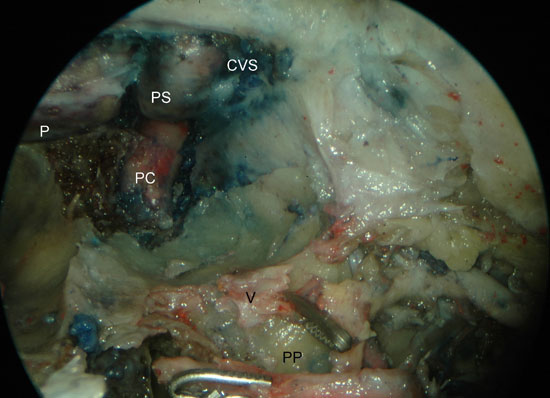
Figure 5. Left PPF approach. Identification of the vidian nerve after partial drilling of the sphenoid floor. A) initial identification of the vidian nerve. B) after partial removal of the pterygoid process. PC, paraclival segment of the internal carotid artery; PS, parasellar segment of the internal carotid artery; P, pituitary; CVS, cavernous sinus; V, vidian nerve; ION, infraorbital nerve; SPA, sphenopalatine artery; IOA, infraorbital artery; PN, greater palatine nerve; PP, pterygoid process.
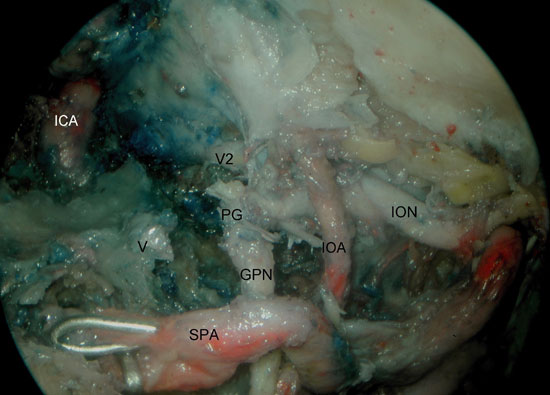
Figura 6A
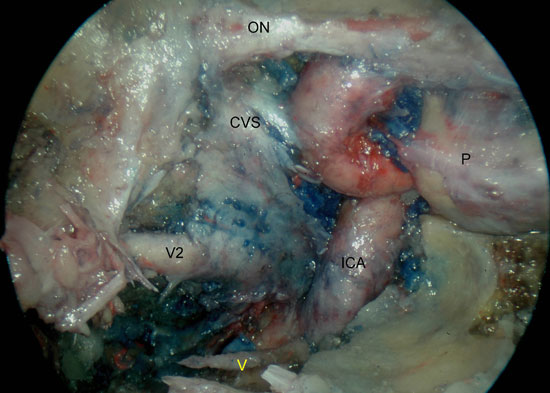
Figure 6. A) Right sphenoid sinus, isolation of the vidian canal and anterior genu of the ICA. B) Left sphenoid sinus, identification of the foramen ovale, mandibular nerve, petrous portion of the ICA and middle cranial fossa. V2, maxillary nerve; ICA, internal carotid artery; P, pituitary; CVS, cavernous sinus; ON, optic nerve; V, vidian nerve; MCF, middle cranial fossa; V3, mandibular nerve; ICA, petrous segment of the ICA; M, Meckel's cave.