IntroductionEpidemiological studies point to vocal nodules as being the main causes of dysphonia in children, which is directly related with vocal abuse 1-5. However, even though nodules are the most frequent in incidence, in clinical practice we have found other laryngeal lesions responsible for dysphonia in children, such as cysts, bridges, sulcus vocalis, and if not properly diagnosed, they can justify therapeutic failure. These lesions named by Pontes et al.6 as minimal structural alterations correspond to small congenital abnormalities of the laryngeal structural configuration or anatomical deviations whose diagnosis requires careful examination of the mucosa cover of the vocal folds. Many of these lesions can be diagnosed in children, being responsible for a large percentage of dysphonia cases in children 6.
As a result of technology advance in videoendoscopy in the past decades, diagnosis of pediatric dysphonia is getting better, which allows consequent better therapeutic management 7. Endoscopic examination provides detailed analysis of vocal folds and allows us to observe small lesions or even imperfections of the mucosa cover, which used to be generically diagnosed as vocal nodules. However, in many cases, even after nice and clear endoscopic images, diagnostic doubts persist, and in some cases owing to lack of collaboration of the child during the exam, or because diagnosis is difficult and requires palpation and exploration of the vocal folds with delicate instruments, it requires the indication of direct laryngoscopy under general anesthesia to clarify the diagnosis. This situation will be favored by the inclusion of rigid and contact endoscopy, when made available to such procedures 8.
The question about the best therapeutic management in pediatric dysphonias always follows professionals that work in the area. In many cases, especially when there is hypothesis of laryngeal minimal structural alteration, the indication of direct laryngoscopy under general anesthesia will be nothing more than a more detailed examination of the vocal folds, without the conduction of any surgical procedure. In these cases, the parents should be informed about all possibilities.
The present study aimed at analyzing retrospectively the ENT and endoscopic assessment of dysphonic children seen at the Ambulatory of Phoniatrics, Medical School of Botucatu, for the past 5 years, in order to analyze gender prevalence, age, main diagnosis of dysphonia, management adopted and clinical evolution.
MATERIAL AND METHODWe conducted a retrospective study of the medical charts of 71 children (45 boys and 26 girls), aged 3 to 13 years, with main complaint of vocal disorder, followed up at the Ambulatory of Phoniatrics, Discipline of Otorhinolaryngology, Medical School of Botucatu, Unesp, for the past 5 years. We excluded children with functional dysphonia and those whose main complaint was respiratory disorder as well as those with laryngeal papillomatosis, glottic or sublogttic stenosis or vocal fold bilateral paralysis.
We analyzed gender prevalence, age range, diagnosis of dysphonia (based on the findings of indirect laryngoscopy, videoendoscopy and direct laryngoscopy), symptoms associated with nasal obstruction (oral breathing, night snoring, repetitive rhinosinusitis, nasal allergy, etc.), the selected treatment and clinical progression.
All children had been submitted initially to indirect laryngoscopy and laryngeal endoscopy examination using telelaryngoscopy or flexible nasoendoscopy, and the latter was reserved to children that had not allowed telescope use. We used a rigid telescope of 70o and 7mm diameter, brand Machida, model C4-C30. Images were recorded using a microcamera coupled to the endoscope (brand Olympus, model A10 -T2), halogen light source (brand Olympus, model CLV-U20), video monitor (brand Sony 14 inches), videocassette (brand Sony, model SVO - 1450) and tape VHS, to record the examination.
Children that did not allow the examination by telescope were submitted to flexible nasofiberoptic examination, brand Olympus, 3.5 mm diameter, with topical anesthesia of the nasal fossae and oro and hypopharynx.
In cases in which the diagnoses were doubtful, even after videoendoscopy, we indicated direct laryngoscopy under general anesthesia to confirm intraoperative diagnosis.
In children that presented nasal obstruction symptoms associated with general case of dysphonia, we analyzed the findings of the ENT general examination, the results of the previous endoscopic exams that they had in the medical charts, the results of paranasal sinuses and rhinopharynx x-rays and surgical procedures and clinical treatments that they had already been submitted to.
In the statistical analysis of data concerning the diagnoses found we used the chi-square test. For the comparison between genders and gender prevalence concerning the diagnoses we used Goodman's test. The level of significance used was 5% (p<0.05).
RESULTSWe assessed 71 children, being 45 boys and 26 girls. The age range comprised 7 children aged between 3 and 6 years, 48 children aged between 6y1m and 10 years, and 16 children aged between 10y1m and 13 years. Telescopic examination was conducted in 59 children and nasofiberoptic examination in 12 children.
The main diagnoses found were: vocal nodules (47 cases; 66.2%); vocal cysts, both epidermic (7 cases, 9.9%) and fistulated (6 cases, 8.4%); sulcus vocalis (5 cases; 7.1%), mucosa bridges associated with cysts (2 cases; 2.8%); anterior commissure microweb associated with vocal nodules (2 cases; 2.8%), and vocal polyps (2 cases, 2.8%) (Table 1).
The definite diagnoses of the lesions as fistulated cysts (4 cases), mucosa bridge (2 cases) and epidermic cyst (3 cases) were only confirmed through direct laryngoscopy under general anesthesia, which was not possible in children that refused to undergo the exam.
Table 2 analyzes the relation between diagnosis of pediatric dysphonia and gender prevalence. There was greater incidence of dysphonia in boys, especially of vocal nodules (X2= 9.90; p<0.05).
Table 3 sums up the main therapeutic approaches adopted in view of the diagnoses, as well as clinical outcome. It was considered favorable when there was clinical improvement and/or improvement of the endoscopic aspect from the initial laryngeal lesion; the opposite was considered unfavorable.
As to associated nasal factors, through the review of medical charts, it was possible to detect that 19 children with vocal nodules presented associated complaints of nasal obstruction. Out of the total, 11 presented adenotonsillar hypertrophy (diagnosis confirmed by physical examination and rhinopharynx radiological exam), being that 10 of them had been submitted to tonsil surgery and one child was still waiting for the surgery. Allergic rhinitis was diagnosed in 8 children, and they were submitted to drug clinical treatment. Two children that presented vocal fold nodules presented associated allergic rhinitis and were under clinical treatment with intranasal corticoid therapy.
Table 1. Main diagnosis of dysphonia observed in the 71 children analyzed.

X2 = 152,82 p= 0,001
Table 2. Relation of diagnosis of pediatric dysphonia and gender.

X2=9,90 p<0,05
Table 3. Childhood dysphonia: clinical treatment and evolution.
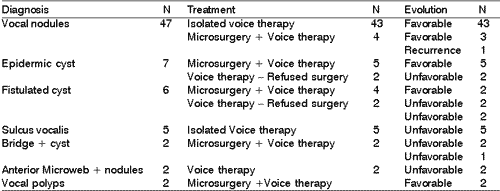
According to our results, as well as other authors', the episodes of pediatric dysphonia are more frequent among boys aged 6 to 10 years, especially the vocal nodules. Nodules correspond to epithelial thickness, normally bilateral, located in the junction between the middle third of the free margin of the vocal folds, and they result from constant mechanical trauma of the vibration portions of the vocal folds 1-4 (Figure 1).
Among the etiologies for the development of nodules we can include vocal abuse and psychoemotional factors. Exaggerated and inappropriate use of voice is a constant report of these children's parents, as well as the emotional profile of an anxious, agitated, aggressive and hyperactive child. However, the complaints of nasal obstruction associated with vocal nodules have been highlighted by some authors among the predisposing factors for the development of vocal nodules, a fact that has also been constant in the present study 3, 9, .10. In such situations, the possible factors that participate in the pathophysiology of the development of lesions include: inflammatory response of laryngeal mucosa to allergic factors of upper airways in atopic patients, and inhalation of poorly conditioned air, forced by permanent oral breathing, predisposing to dryness of airway mucosa and inhalation of non-filtered micro particles through the nasal fossae, facilitating recurrent infections in the upper airways. Moreover, we should emphasize that the resistance imposed by oral breathing and regulated by nasal reflexes participate in the regulation of the appropriate expansion of the thoracic cavity and alveolar ventilation, resistance that is absence in oral breathing 9. Other factors that have been reported in the origin of vocal nodules are anatomical laryngeal abnormalities (microweb in anterior laryngeal commissure), gastroesophageal reflux and hormone disorders. Therefore, we can say that the origin of nodules is multifactorial.
In vocal nodules, the treatment of choice is vocal reeducation, however, for speech therapy to succeed, it is important to have discipline and awareness by the children and family members concerning daily activities. Surgical indication is reserved for cases that are resistant to treatment, those in which there are questions concerning differential diagnosis with vocal cysts (4 cases in our sample) and when they are associated with congenital lesions such as minimal structural alterations 2.
Minimal structural alterations of the vocal folds were named such in 1994 by Pontes et al.6 as "a group of minor congenital abnormalities whose impact, if present, is restricted to laryngeal phonation ranging from simple anatomical variations to minor congenital malformations". To the authors, they can be classified into 3 groups: laryngeal asymmetry, deviation of glottic proportion and alterations of the vocal fold cover. They include vasculodysgenesia, closed cyst (Figure 2), open cyst, mucosa bridge (Figure 3), sulcus vocalis (Figure 4) and laryngeal web or laryngeal microweb (Figure 5). Depending on the extension of the damage, the presence of abnormalities can cause damage to vocal quality. The studies that describe them caution us about the need to have precise diagnosis of the lesion using detailed examination of the larynx, allowing its diagnosis even in pediatric larynges 6. These lesions were detected in our sample, amounting to 31% of the cases, incidence slightly below that reported by Madruga de Melo et al.7, who observed an incidence of 37.5% among dysphonic children assessed by them.
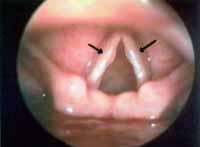
Figure 1. Bilateral vocal nodules (arrows). Telelaryngoscopy.
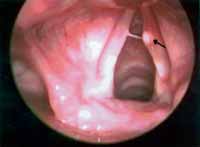
Figure 2. Cyst on the right vocal fold (arrow). Telelaryngoscopy.
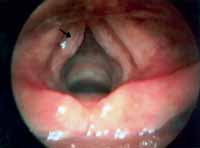
Figure 3. Mucosa bridge and left vocal fold cyst (arrow). Telelaryngoscopy.
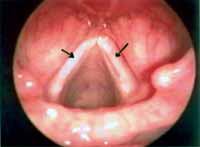
Figure 4. Bilateral sulcus vocalis (arrows). Telelaryngoscopy.
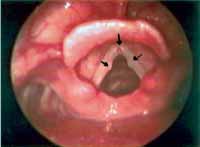
Figure 5. Vocal nodules (lateral arrows) and microweb in the anterior commissure (inferior arrow). Telelaryngoscopy.
Vocal cysts correspond to epithelial lesions, deep into the lamina propria of the vocal folds, filled with content, and recovered by stratified epithelium. The epidermic cysts are originated from epithelium ectopia and have a pearl-like appearance; glandular cysts correspond to obstruction of the serous or mucous excreting duct 11. In the case of vocal cysts, when vocal impact is important, surgical management is the best option, since they tend to increase in size or even drain its content through a mucosal fistula. In cyst microsurgery we can delicately dissect them, even if they are fistulated, with good surgical outcome 11-14.
As observed in the progression of the cases reported here, some children who had vocal fold cyst and refused surgery did not progress well only with speech therapy. Even though vocal reeducation reduced vocal impact, the presence of permanent organic lesion of the vocal fold mucosa prevented complete success of therapy.
In our sample we diagnosed 5 cases of sulcus vocalis. Sulcus vocalis corresponds to congenital malformations in which we observe sulcus or depression in the epithelium of the free margin of one or both vocal folds. The bottom part of the sulcus is adhered to the vocal ligament, explaining mucosa rigidity, responsible for poor vocal quality 6, 15-17. Voice is harsh, high and breathy. The degree of glottic insufficiency, characterized by the anterior-posterior spindle chink depends on the extension and depth of the lesion. Treatment with speech therapy is disappointing, especially in cases of wide spindle chinks.
Using surgical treatment of sulcus vocalis, we can have good vibration of the mucosa and reduction of the glottic chink. Among the surgical proposals that have gained highlight we can include removal of the sulcus in its whole extension, releasing the atrophic mucosa 13, 16, intracordal injection of collagen or fat 18, introduction of muscle fascia 19, and techniques of multiple sections or stripping 20. None of the surgical approaches has reached perfect vocal quality, but they manage to reduce vocal strain and fatigue thanks to the reduction of glottic insufficiency and detachment of the mucosa from the vocal ligament. Since the children with sulcus vocalis in our sample were below the age of 10 years, we decided to postpone the decision to indicate surgery up to adolescence or adult age, since the success of the surgery is not guaranteed. Therefore, there was no improvement in vocal quality only with voice therapy in these cases.
Mucosa bridge corresponds to a mucous arch detached from the vocal fold. It can be positioned close to the free margin or the vestibular surface. When there is vocal impact it should be removed 6. It is almost always associated with other lesions such as cysts or sulcus vocalis and in these cases, it can hinder the success of treatment, as has possibly occurred in one of our children that presented more than one associated minimal structural lesion.
We found two children with vocal nodules associated with microweb in the anterior glottic commissure. This association had already been reported by Ford et al.21 in whose publication the authors stated that the association was the reason for failure in vocal therapy, suggesting the need for surgical removal of the microweb. The therapeutic results with isolated therapy in our cases were not satisfactory either.
Vocal polyps are rare in children, since they are chronic inflammatory reactions to vocal fold mucosa, much more common in adults. Two children in our sample had diagnosis of polyps and presented complaints of nasal obstruction by allergic rhinitis, factors responsible, in many occasions, for the origin of the polyps. The surgical management associated with voice therapy resulted in favorable progression, with normalization of vocal patterns in both children.
CONCLUSIONVocal nodules were the main causes of dysphonia in the studied children, being more frequent in male subjects. In these cases, voice therapy was the management of choice and it proved to be effective.
Minimal structural lesions such as cysts, sulcus vocalis, bridges and microwebs were responsible for a large percentage of pediatric dysphonias in our sample and for the unfavorable results in treatment. The definite diagnoses of these lesions in children's larynges require careful and detailed endoscopic examination, many times under general anesthesia.
REFERENCES 1. Verhulst J, Le Huche F, Marvaud J. VII Rencontres de l'Institut G. Portmann: Nodules et formations paranodulaires. Rev Laryngol 1993; 114:251-7.
2. Cervantes O, Abrahão, M. O nódulo vocal - conceitos atuais. Rev Br Med 1995; 2:12-7.
3. Gray SD, Smith ME, Schneider H. Voice disorders in children. Ped Clin Nor Am 1996; 43:1357-84.
4. Sarfati J, Auday T. Evolution des dysphonies bénignes de l'enfant. Rev Laryngol Otol Rhinol 1996;117:327-9.
5. Dejonckere PH. Voice problems in children: pathogenesis and diagnosis Inter J Ped Oto Rhino Laringol 1999; 49:311-4.
6. Pontes P, Belhau M, Gonçalves, M. I. Alterações estruturais mínimas da laringe (AEM): considerações básicas. Acta Awho 1994; 12:2-6.
7. Melo ECM, Brasil OCO, Melo DM, Leventi NM, Brito LL. Alterações estruturais mínimas da cobertura das pregas vocais em crianças. Arq Otorrinolaringol 2002; 6:115-9.
8. Andrea M, Dias O, Santos A. Contact endoscopy of the vocal cord. Normal and Pathological Patterns. Acta Otolaryngol (Stockh) 1995; 112: 314-6.
9. Meirelles RG. Obstrução nasal e nódulos vocais. Rev Bras Otorrinolaringologia 2001; 67:387-92.
10. Mori K. Vocal fold nodules in children: a preferable therapy Inter J Ped Oto Rhino Laringol 1999; 49:303-6.
11. Steffen N. Cistos de pregas vocais: análise de 96 casos. Rev Bras Otorrinolaringologia 1995; 61:179-86.
12. Bouchayer M, Cornut G, Witzig E et al. Epidermoid cysts, sulci, and mucosal bridges of the true vocal cord: a report of 157 cases. Laryngoscope 1985; 95:1087-94.
13. Bouchayer M, Cornut G. Microsurgical treatment of benign vocal folds lesions. Folia Phoniatr 1992; 44:155-84.
14. D'Ávila JS, Santos Júnior RC, Neves AB. Variação técnica (microcirúrgica) para cisto fistulizado de prega vocal. Rev Bras Otorrinolaringologia 1999; 65:131-9.
15. Hirano M, Yoshida T, Tanaka S, Hibi S. Sulcus vocalis: functional aspects Ann Otol Rhinol Laryngol 1990; 99:679-83.
16. Ford CN, Inagi K, Kridr A et al. Sulcus vocalis: a rational analytical approach to diagnosis and managment. Ann Otol Rhinol Laryngol 1996; 105:189-200.
17. Rock JB, Bouchayer M, Cornut G. Le sulcus des cordes vocales. JFORL 1992; 138-44.
18. Ushijima T, Kase Y, Honda K et al. Effectiveness of intracordal injection for sulcus vocalis. Folia Phoniatr 1986; 38:365-8.
19. Tsunoda K, Takanosawa M, Niimi S. Autologous transplantation of fascia into the vocal fold: new phonosurgical technique for glottal incompetence. Laryngoscope 1999; 109: 504-8.
20. Pontes P, Belhau M. Treatment of sulcus vocalis: auditory perceptual and acoustical analysis of the slicing mucosa techinique. J Voice 1993; 4:365-76.
21. Ford CN, Bless DM, Campos G e. al. Anterior commissure microwebs associated with vocal nodules: detection, prevalence and significance. Laryngoscope 1994; 104:1369-75.
1 Ph.D., Assistant Professor, Discipline of Otorhinolaryngology and Head and Neck Surgery, Medical School, Botucatu (UNESP).
2 Resident Physician, Discipline of Otorhinolaryngology and Head and Neck Surgery, Medical School, Botucatu (UNESP).
Study conducted at the Discipline of Otorhinolaryngology and Head and Neck Surgery, Medical School, Botucatu (UNESP).
Address correspondence to: Regina Helena Garcia Martins - Faculdade de Medicina de Botucatu (UNESP) -
Departamento de Oftalmologia, Otorrinolaringologia e Cirurgia de Cabeça e Pescoço Botucatu SP 18618-970.
Tel (55 14) 6802-6256 - E-mail: rmartins@fmb.unesp.br
Article submitted on February 06, 2003. Article accepted on April 04, 2003.


