IntroductionThe Otorhinolaryngologists that perform laryngeal microsurgery using laser CO2 in addition to knowing anatomy, histology and physiology of human larynx in detail should know the physical and biological principals of laser technology and its interaction with the tissues.
Laser is a luminous flow of high intensity energy, which is characterized by parallelism of its waves (coherent light) and its narrow spectral distribution with the same wavelength (monochromatism). Through lenses, prisms and mirrors, the light can be concentrated and projected with high concentration of energy in small and precise areas3, 4.
CO2 laser is a mixture of gases of CO2, N2 and H2 whose wavelength is 10.6 micron in the infrared spectral range. The caloric energy is produced by a beam that is almost completely absorbed by biological tissues, producing tissue destruction by the quick vaporization of water in the tissues and thermal denaturation of its tissue proteins. The first factor is responsible for the ablation of tissues, whereas protein denaturation in a small number of surrounding cells obliterates the small vessels and coagulates instantly the blood in the vessels up to 0.5mm in diameter 5, 6, 7.
The advantages of laser CO2 interaction with organic tissues in relation to conventional surgical methods are: reduction of bleeding resultant from photocoagulation of vessels of equal caliper or inferior to half millimeter, reduction of postoperative edema that occurs owing to small manipulation of the surgical area, easiness of access to the surgical field, precision with possibility of directing the laser to the desired area, reduction of surgical time and quick recovery 7. In addition, scarring after CO2 laser is normal or has minor delay, causing complete epithelialization in three weeks 8.
The use of microspot, reducing the diameter of the laser beam in the impact area associated with super-pulse, in which energy is applied in a shorter time, allows the beam to act as a delicate knife, making precise incisions with fewer thermal effects over the vocal ligament, without carbonization and with considerable reduction of edema and fibroblastic proliferation 10, 11.
Excessive thermal energy transmitted to the vocal folds can result in fibrosis and adherence to the vocal ligament epithelium, creating rigidity and mucosa wave abnormality and impairing vocal quality. It used to happen with the older generations of laser, which used spots greater than 800 micron and vaporization technique of benign lesions.
The idea of phonomicrosurgery created the concept that non-intentional transmission of thermal energy of CO2 laser over the superficial lamina propria results in fibrosis and adherence of the vocal fold epithelium over the vocal ligament. The adhered vocal fold mucosa creates a vocal system of tension and impairs vocal production 11-14.
As a result of the thermal effects over the vocal folds the use of laser for surgical therapy of benign vocal fold lesions is still controversial 13-16. The use of CO 2 laser for the treatment of benign vocal fold lesions depends on cases that are properly selected and the experience of the surgeon with modern technology 17-10.
CO2 laser with microspot is effective and safe when used appropriately at intermittent low levels, to remove the benign laryngeal lesions. Refined microlaryngeal instruments can promote equivalent results when used in small pediculated lesions, such as nodules. The laser technique is particularly indicated for the treatment of patients that have vascular polyps and lesions such as granulomas. In addition, laser is extremely useful in incisions and removal of polypoid vocal folds with mixoid stroma 16.
The advantage of laser concerning hemostasis allows easier dissection of microflaps. Cold instruments are normally better used for the resection of superficial and small lesion. CO2 laser facilitates the resection of large and vascular lesions, as well as those in the supraglottic regions 18.
By analyzing the histology and quantitative amount of collagen in the vocal folds of dogs in the control group and the group submitted to incision with CO2 laser and cold instruments, our study intended to: analyze the amount of collagen, compare quantitatively and statistically the collagen of the dog vocal folds in groups submitted to surgery with cold instruments and laser CO2 and evidence whether there is statistical difference between the control group and the groups submitted to surgery with cold instruments and CO2 laser.
Material and MethodsWe analyzed the vocal folds of 17 dogs that ranged from 6 to 13 kg of weight. Three dogs were part of the control group and 14 were submitted to surgical procedures, which followed the same standards. All 14 dogs were submitted to general anesthesia before the procedures. They were treated with pre-anesthetic Ketalar® (ketamine) intramuscularly 50 grams per body weight in kg.
The 14 dogs were anesthetized with intravenous Thionembutal® (sodium thiopental) 40 mg/Kg/weight and using laryngoscope Kleinsasser, we performed suspension laryngoscopy. The dog vocal folds were visualized through optical binocular straight microscope (DF Vasconcelos) with 400mm lenses.
The middle third of the vocal fold on the left of the 14 dogs received an incision of about 5.0 cm with cold instrument - a knife. The right vocal fold received an incision of about 5.0cm with CO2 laser (Sharplan® - Israel) of 1-watt continuous power in focused mode Acuspot® of 250 micron.
Thirty days after surgery, they were sacrificed with intravenous injection of Thionembutal® and 10 ml of potassium chloride 19.1%, and laryngectomized. Dog larynges were removed and placed in formalin 10%.
The vocal folds of the 17 dogs after formalin 10% immersion were washed with water and dehydrated in solutions with increasing concentrations of ethanol, diaphanized in xylol and placed in paraffin.
We made 3-micron sections, stained with solution of 0.1g of Sirius Red in 100 ml of picric acid solution and prepared them in glass slides for histochemical analysis.
The slides were measured through the total area of tissue obtained and the birefringent area, which corresponded to the total amount of collagen.
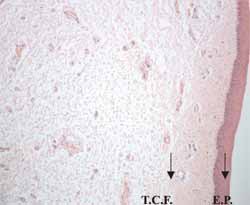
Figure 1 - Histology sections of the canine vocal fold - (Hematoxylin - Eosin).
The vocal fold is recovered by non-keratinized pavement stratified epithelium (E.P.). Below the epithelium we can see the lamina propria formed by loose connective tissue containing blood vessels and diffuse lymphatic tissue (T.C.F.).
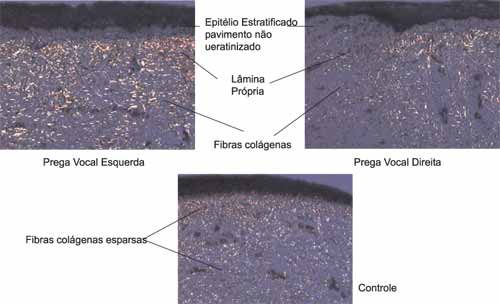
Figure 2. Comparison between the right and left vocal folds of one of the 14 dogs of the group submitted to surgery with one vocal fold of a dog in the control group.
In 14 dogs submitted to CO2 laser procedures and cold instrument incision we quantified collagen and analyzed its percentage. In the 3 control cases we also analyzed the amount of collagen and the percentage both in the right and left vocal folds. In all analyses, the total area checked by the pathologist was constant, of 12,55010 squared micron.
There were lower amounts of collagen in the right vocal folds (submitted to incision with CO2) than in the left vocal folds (submitted to incision with knife) (Figure 2).
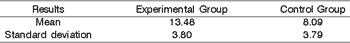
In the middle third of the vocal folds of dogs submitted to incision with CO2 laser, we observed an area of collagen that ranged from 82168,02 to 20092 mi2 and percentage of collagen that ranged from 6,54 to 16,007. The mean for collagen percentage was 9.98 in these vocal folds (Table 1).
In the middle third of right vocal folds in animals submitted to knife incision we observed area of collagen that ranged from 96344,01 to 252809,20 mi2 and collagen percentage that ranged from 7,67 to 20,14. The mean percentage of vocal fold was 13,48 (Table 2).
The collagen area of the right vocal folds in animals in the control group ranged from 43322,95 to 296363,10 mi2 and the collagen percentage ranged from 3,45 to 23,61. The mean percentage of collagen for these vocal folds was 8.56 (Table 3).
The collagen area of the left vocal folds in animals in the control group ranged from 36957,53 to 164978,60 mi2 and the collagen percentage ranged from 2,94 to 13,1456. The mean percentage of collagen in left vocal folds of the control group was 8,08 (Table 3).
Statistical Analysis of Results
In order to check the existence of difference between the results obtained in the right and left vocal folds, that is, to check whether the percentage of collagen in the left vocal fold was significantly greater than the collagen percentage in the right vocal fold we applied the mean difference test (t test).
We found t = 2,713, which comparing to the table values (t = 1,706 for p = 0,05 and t = 2,479 for p = 0,01) led us to the conclusion that the collagen percentage was significantly greater both for the level 0.05 and 0.01 of the group submitted to knife incision.
Upon analyzing the results separately, that is, the vocal folds submitted to laser CO2 and the vocal folds of the control group, we could notice the results that follow.
Applying the t test, we found t = 0,64, which comparing to the table values (1,721 for p = 0,05 and 2,518 for p = 0,01), led us to the conclusion that there was no statistically significant difference between the percentage of collagen in the vocal fold of animals in the experimental and the control groups.
However, we observed statistical results in dogs submitted to incision with knife and the dogs in the control group and concluded what is shown in Table 6.
The t test for the difference of means provided t = 3,287, which compared to the values of the table (1,721 for p = 0,05 and 2,518 for p = 0,01) showed there was significant difference between the percentages of collagen in the left vocal fold of animals in the experimental group and the control group animals. The percentage of collagen in the left vocal fold of animals in the experimental group was significantly greater than the percentage of collagen in the left vocal fold of animals of the control group.
Table 1. Area and percentage quantity of collagen of dogs submitted to laser CO2 incisions (right vocal fold).
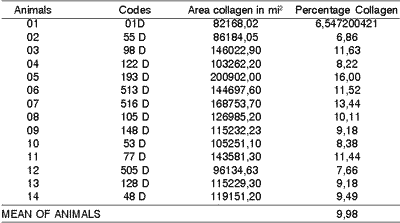
Table 2. Area and percentage quantity of collagen in dogs submitted to incision with cold instruments (left vocal fold).
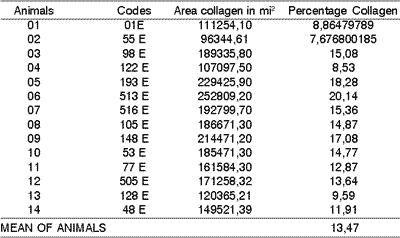
Table 3. Area and percentage quantity of collagen in the vocal folds of the control group.
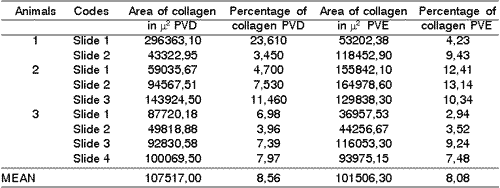
PVD: Right vocal fold; PVE: Left vocal fold.
Table 4. Animals of the experimental group - Percentage of collagen.

Table 5. Statistical result of the mean of collagen and standard deviation on the right vocal folds of the group submitted to CO2 laser surgery and the Control Group.
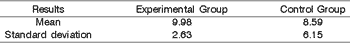
Table 6. Statistical result of the mean of collagen and standard deviation of the group of animals submitted to incision with cold instrument and the Control Group.
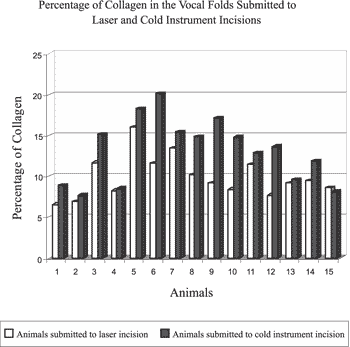
Graph 1. Percentage of collagen in the vocal folds submitted to incision with cold instruments and laser CO2 in 14 dogs. Dog 15 represented the mean percentage of collagen in 3 dogs that had not been submitted to any procedure.
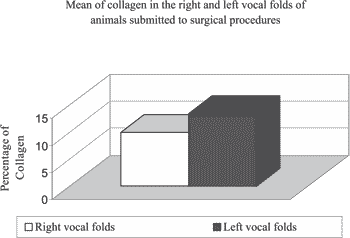
Graph 2. Mean of collagen in right vocal folds submitted to CO2 laser incision and left vocal folds submitted to cold instrument incision.
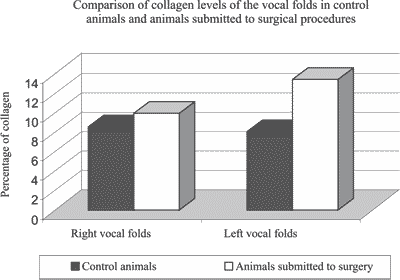
Graph 3. Mean of collagen between the right and left vocal folds of control animals and left vocal folds submitted to surgery.
It is known that collagen is present fundamentally on the deepest layer of the lamina propria and it increases in quantity as we go deeper towards the vocal ligament and muscle 12. Reinke's space presents only a small amount of collagen fibers. The amount of fundamental fibers that serve to promote resistance to tissue tension is essential in the observation of scarring of the vocal folds after surgical procedure.
The importance of this study resides in the quantification of collagen, since the greater the vocal fold vibration and the greater the amount of fibers, the greater the resistance and, consequently, the higher the damage to vocal production. The method of Picrosirius, together with polarization microscopy, demonstrates more clearly the collagen fibers 21-25.
In the study we observed that the amount of collagen in the vocal folds submitted to different types of surgery, both with CO2 laser and cold knife, presented statistically significant difference, as observed by Tables 1 and 2. The presence of collagen was significantly higher in the vocal folds submitted to incision with knife than those submitted to CO2 laser, which allowed the conclusion that the impact caused on vocal production is lower in the surgery with laser when we use low power and small microspot.
According to some authors, CO2 laser associated with microsurgery techniques is efficient to make better hemostasis than cold instrument techniques and the performance of microflaps dissection is easier 16. Other authors showed that there is improvement in vocal function in patients submitted to techniques with cold knife and laser CO2 17.
Benninger19, in a clinical study, performed a comparison between CO2 laser and cold instruments in benign laryngeal lesions and concluded that there is no postoperative clinical difference between the above-mentioned techniques when microdissection of vocal fold nodules, polyps and cysts of mucous retention is performed.
The ideas by Ishiki11 about the effects of thermal energy with laser CO2 causing adherence of vocal fold mucosa, increasing tension, with vocal impairment, can be avoided by using modern technology with microspots of 250 micron, lower power and super-pulse 9, 10. It allows further indications of surgeries with laser CO2 for benign vocal fold lesions16,17,19,20.
ConclusionLaser CO2 is the most used laser in surgeries of Otorhinolaryngology and Head and Neck Surgery. The new laser technologies, such as reduction of microspot and use of super-pulse, have increased the indications of its use to benign vocal fold lesions, respecting the microsurgical techniques.
This pioneer study showed that the amount of collagen of vocal folds submitted to surgical procedures was greater than in the control group, the amount of collagen was statistically greater in the animal group submitted to cold instrument incision than with CO2 laser, and there was no statistically significant difference concerning the control group and the group submitted to laser CO2 incision.
Therefore, laser CO2 is a safe and effective therapeutic method when using the modern technology and the principles of phonomicrosurgery, without causing increase in vocal fold tension, by observing the amounts of collagen.
REFERENCES 1. Jako GJ & Strong MS. Laser surgery in the larynx: early clinical experience with continuous CO laser. Ann Otol Rhinol Laryngol 1972; 81: 791-8.
2. Strong, MS, Jako GJ et al. Laser Surgery in the Aerodigestive Tract. Am J Surg 1973; 126: 529-33.
3. Andrews AH & Polanyi TG. Microscopic and endoscopic surgery with the CO laser. Bristol: John Wright & Sons Ltd.; 1982.
4. Stellar S, Polanyi TG, Bredemeier HC. Laser in surgery. In: Wolbarsht ML. Laser applications in Medicine and Biology. New York: Plenum Publishing Corp 1973; 2: 241-93.
5. Maiman TH. Stimulated optical radiation in ruby. Nature 1960; 187: 493.
6. Polanyi TG, Bredemeier HC, Davis JR. Lasers for surgical research. Med Biol Eng 1970; 8: 541-8.
7. Pinto JA. Microcirurgia com Laser de CO e suas Aplicações em Otorrinolaringologia. Revista Brasileira de Otorrinolaringologia 1986; 52: 26-35.
8. Mihashi S & Jako GJ et al. Laser surgery in otolaryngology: interaction of CO laser and soft tissue. Ann NY Acad Of Sciences 1976; 267: 263-94.
9. Ossof R H, Coleman JA, Courey MS, Duncavage JA, Reinisch L, Wekhaven JA. Clinical applications of laser in otolaryngology - head and neck surgery. Laser in Surgery Medicine 1994; 15: 217-48.
10. Ossof RH, Reinisch L. Laser Surgery. In: Ossof RH. The head and neck Nashville Vanderbilt Press 1997; 36: 678-94.
11. Isshiki N. Phonosurgery: theory and practice. Tokyo: Springer-Verlag 1989; 7-11.
12. Hirano M. Phonosurgical Anatomy of the Larynx. In: Ford CN, Bless DM. phonosurgery: assesment and surgical management of voice disorders. New York: Raven Press 1991; 3: 25-41.
13. Hirano M, Hirade Y, Kawasaki H. Vocal function following carbon dioxide laser surgery for glottic carcinoma.Ann Otol Rhinol Laryngol 1985; 94 (3): 232-5.
14. Burian K, Hofler H. Zur mikrochirurgischen therapien von Stimmbandcarcinomen mit dem CO laser. In: Functional results after CO laser surgery compared with conventional phonosurgery. T J of Laryngol and Otol 1999; 113: 140-4.
15. Sataloff RT, Spiegel JR, Hawkshaw M, Jones A. Laser surgery of the larynx: the case for caution. Ear Nose and Throat Journal 1992; 71: 593-5.
16. Remacle M, Lawson G, Watelet JB. Carbon dioxide laser microsurgery of benign vocal fold lesions: indications techniques and results in 251 patients. Ann Otol Rhinol Laryngol 1999; 108: 156-64.
17. Hormann K, Baker-Schreyer A, Keilmann A, Biermann G. Functional results after CO laser surgery compared with conventional phonosurgery. The J of Laryngol and Otol 1999; 113: 140-4.
18. Zeitels SM. Laser versus cold instruments for microlaryngoscopic surgery. Laryngoscope 106 (5): 545-52.
19. Benninger MS. Microdissection or microspot CO laser for limited vocal fold benign lesions: a prospective randomized trial. Laryngoscope 2000; 110: 1-17.
20. Sittel C, Eckel HE, Eschenburg C. Phonatory results after laser surgery for glottic carcinoma. Otolaringol Head and Neck Surg 1998; 119: 418-24.
21. Junqueira LC. Differencial staining of collagens type I II and III by sirius red and microscopy. Arch Histolol JPM 1978; 41: 267-74.
22. Junqueira LC, Carneiro. Histologia básica. 9a Ed. Rio de Janeiro Guanabara Koogan 1999; 5: 69-93.
23. Lapière CM. The ageing dermis: the main cause for the appearence of old skin. Br J Dermatol 1990; 122: 5-11.
24. Junqueira LC. Picrosirius staining plus polarization microscopy a specific method for collagen detection. Histochem J 1979; 11: 447-55.
25. Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the picrosirius-polarizations methods. Histochem 1982; 74: 153-6.
1 Preceptor of Medical Residence, Núcleo de Otorrinolaringologia de São Paulo.
2 Joint Professor, Medical School of Ribeirão Preto, University of Sao Paulo.
3 Resident Physician, Núcleo de Otorrinolaringologia e Cirurgia de Cabeça e Pescoço de São Paulo.
4 Ph.D., Professor, Department of Cellular and Molecular Biology and Pathogenic Bioagents, FMRP-USP.
5 Director of Núcleo de Otorrinolaringologia e Cirurgia de Cabeça e Pescoço de São Paulo (NOSP).
6 Ph.D., Professor of Post-graduation, Medical School, Ribeirão Preto, University of Sao Paulo, Major in Otorhinolaryngology.
Address correspondence to: José A. Pinto - Alameda dos Nhambiquaras, 159 - Moema 04090-010 São Paulo SP.
Tel/Fax (55 11) 5573-1970 - E-mail: japorl@cepa.com.br
Study conducted by the Department of Ophthalmology and Otorhinolaryngology, Hospital das Clínicas, Medical School, Ribeirão Preto -
USP together with Núcleo de otorrinolaringologia e Cirurgia de Cabeça e Pescoço de São Paulo - Hospital São Camilo-SP.
Article submitted on August 01, 2003. Article accepted on September 04, 2003.


