IntroductionOtoacoustic emissions OAE) are sounds generated in the normal cochlea either spontaneously or as a response to acoustic stimulation. Currently, it is considered that OAE reflect the activity of active biological mechanisms in the cochlea and that the outer hair cells are responsible for such mechanisms1.
Otoacoustic emissions are classified into spontaneous and evoked. Spontaneous otoacoustic emissions occur without presentation of a stimulus. Evoked emissions are elicited based on stimulus that can be brief clicks - originating transient evoked otoacoustic emissions, or stimuli of two pure tones presented simultaneously - distortion product otoacoustic emissions.
Therefore, distortion product otoacoustic emissions can be defined as the acoustic energy generated inside the cochlea by the non-linear interaction of two primary pure tones (f1 and f2) applied simultaneously 2. Generally, the tone resultant corresponds to frequency of 2f1-f2 and allows the assessment of the cochlear function by frequency range. With distortion product otoacoustic emissions it is possible to analyze the amplitude of response through the dp-gram, the correlation between amplitude and sound intensity (growth curve) and also the latency of responses through the latency-gram.
In 1998, Quiñonez & Crowford3 defined the latency of DPOAE as the time that the acoustic stimulus takes to reach the generating site of the otoacoustic emissions in the cochlea, be analyzed and return to the external acoustic canal to be recorded. In 1999, John and Picton4 conducted a study and concluded that the acoustic responses to high frequency sounds occurred at shorter latencies than low frequencies. One of the reasons for such findings is explained by the phenomenon of the traveling wave discovered by Bèkesy in 1960.5 According to the theory, the waves caused by acoustic stimuli run through the cochlea and distribute the sound of high frequencies to the regions of the cochlear base and the low frequency sounds to the regions of the cochlear apex. Thus, it is suggested that the latency of response increases in low frequencies since the sound takes longer distance throughout the cochlea. Studies in adults showed that there is a decrease in latency with the increase in sound frequency, ranging from 16.7 ms in 732 Hz to 4.7ms in 6396 Hz 6. In a recent study conducted in Brazil, the authors also found decreasing values ranging from 17ms in 720Hz to 2.6ms in 6445Hz 7.
The latency measure of DPOAE is not well known and there are few studies about its use in clinical practice, but there is evidence in some studies that it may be useful for the analysis of cochlear maturation, since the latency is determined by the progression of the traveling wave. The period of life right after birth of the full term babies has not been studied from a morphological and functional perspective. This is an interesting period that corresponds to the beginning of outer hair cell (OHC) functions and their main modifications (involving thresholds, amplitudes and latency).
There are few studies in this area and there are no normal range criteria for such measures, especially in the newborn. Therefore, the purpose of our study was to characterize the latency measures of DPOAE in full term neonates without hearing risk.
Material amd MethodWe assessed 31 newborns, 15 male and 16 female subjects, all born at full term without delivery or gestation complications and no risk for hearing loss. Initially, the mothers signed an informed consent term at the hospital (University Hospital, University of Sao Paulo), and one week later they came to the Speech and Hearing Unit, University of Sao Paulo, to conduct the test.
The project was analyzed by the Committee of Research Project Analysis, Medical School, University of Sao Paulo, and approved by protocol n° 368/98.
The device used for the assessment was system ILO 292 V5.6 Otodynamics. The assessment was conducted in a silent room while the baby was sleeping.
Initially, all newborns were submitted to the hearing screening using transient otoacoustic emissions to analyze the integrity of the cochlear function and rule out sensorial hearing loss. We considered as criteria for pass the presence of responses superior to 3dB in 1 or 1.5kHz and superior to 6dB in 2, 3, and 4 kHz with reproducibility of responses greater than 50% 8. The babies that passed the screening were submitted to analysis of distortion product latency. Latency was assessed using the phase-gradient method described by Kemp and Brown in 19839. The used procedure was the latency-gram in which the latency is calculated by two-phase points. The frequency of DPOAE ranged based on change of f2. During the change in frequency of f2 the ratio f1/f2 ranged successively in steps of f1/f2=1.19-1.21; f2'/f1=1.21-1.22. Latency was then calculated as a change in phase in degrees - 360o ranging in frequency Hz is equal to the drop of the line that connected the two points in phase.
The assessed frequencies were 3 and 6 kHz and the intensity used was 70dB for both primary frequencies. Owing to the high level of noise of the newborns in the lowest frequencies, it was not possible to collect data in frequencies below 3kHz. After the collection, the data were analyzed statistically using the t Student test and significance was analyzed concerning the difference in latency by sound frequency, gender and ear side.
ResultsInitially, we tried to check whether values of the latency of distortion product otoacoustic emissions ranged in function of sound frequency. Therefore, we calculated the means and standard deviation of values of latency for each sound frequency.
The results obtained showed the reduction of latency with increase of sound frequency in both genders and both ears as shown in Graphs 1, 2, 3 and 4.
In the statistical analysis we tried to observe whether there was significance in the decrease of latency with increase of sound frequency. To that end, we applied the t Student test for matched data, comparing the frequencies of 3KHz x 4KHz, 3KHz x 5KHz, 3KHz x 6KHz, 4KHz x 5KHz, 4KHz x 6 KHz and 5KHz x 6 KHz. There results of the analyses showed statistically significant reduction in latency with the increase in sound frequency, except for frequencies of 3 and 4 kHz, in which the reduction was observed but with no statistically significance.
Next, we tried to check whether there was variation in the values of latency concerning the variable gender. The mean values of latency in ms obtained in sound frequencies of 3 and 6 kHz for male and female subjects are presented in Graphs 1, 2, 3, and 4. The mean values for latencies for male subjects were greater than for female subjects in all frequencies, but there was statistically significant difference between genders only in sound frequency of 3kHz on the right, with p = 0.009 being that the greatest latencies were observed in male gender.
We also tried to check whether latency variables varied as a result of ear side. In the statistical analysis, we did not find statistically significant results concerning values of latency to the right and left ears in any of the genders.
Finally, we tried to compare the values obtained in our study with the values in adults with no hearing disorders. All latency values of DPOAE found in the studied newborns were higher than the latency values reported in adults.
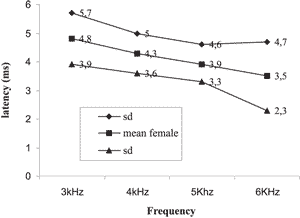
Graph 1. Mean and standard deviation of latency for female subjects on the right
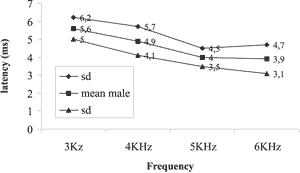
Graph 2. Mean and standard deviation of latencies by frequency for male subjects on the right ear.
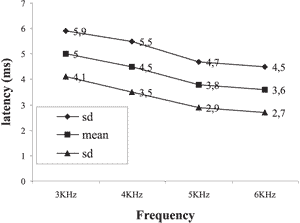
Graph 3. Mean and standard deviation of latencies for female subjects on the left
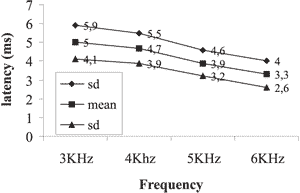
Graph 4. Mean and standard deviation of latencies for male subjects on the left ear
The complete formation of the cochlear anatomy can be observed in the 8th and 9th week of gestation, but the formation of the bone capsule is not complete until the last months of gestation. It is believed that the histology development of the cochlea is not complete before the 6th to 8th month of gestation 10. The first signal of Corti's organ differentiation takes place in the 10th week of gestation. The inner hair cells (IHC) mature before the outer hair cells (OHC). At the end of maturation, OHC present some properties connected with cochlear micromechanisms. In the apical part, the maturation tends to be incomplete (owing to the little efferent innervation). The studies demonstrate an overproduction of OHC during the 15th week of gestation. After this period, the efferent olivo-cochlear system is responsible for the degeneration and formation of some cells. This observation has been correlated with the observation that OAE in babies present wider spectrum than in adults, since newborns have a greater number of OHC. Currently, OAE reflect directly the activities of the OHC and they are being studied to understand the cochlear functions in newborn babies. It is believed that the study of OAE in human beings can serve as good criterion to investigate the physiological events of cochlear maturation 9.
The analysis of results of the present study showed a significant reduction in latency values with the increase in sound frequency. These findings were in agreement with the literature. In all studies analyzed about the topic, we observed a reduction of latency with increase in sound frequency 10-14. This finding is in agreement with the literature that reports a correlation between latency and the cochlear traveling wave described by Bèkesy5. According to this theory, the sound of high frequency produce vibration peaks on the basilar membrane close to the cochlear base, whereas low frequency sounds produce peaks closer to the apex. Such properties are determined primarily by the physical characteristics of the cochlea, which is thinner and more rigid in the base and thicker and more flaccid in the apex 15. Owing to such characteristics of the cochlea, the sound of low frequency tend to take a longer way up to the point of maximum vibration and for this reason, they present higher latency.
Comparing the values obtained in newborn babies with values obtained in adults in a study conducted by Marques in 20007, the newborns presented latency values higher for all tested frequencies. The author found values ranging from 3.5 in 3kHz to 2.4 in 6 KHz for male subjects and 4.3 in 3 kHz to 2.6 in 6 kHz for female subjects.
Brown, in 199414, compared the latency of distortion product otoacoustic emissions in adults and neonates with normal hearing. They observed statistically significant differences in medium frequencies, being the latency in adults higher than in neonates. Since there was no statistically significant difference in high frequencies, the author questioned the cochlear immaturity in high frequencies in neonates. It is believed that latency of DPOAE can be connected with the principle of local sifting place and also owing to formation time of the cochlear filter. The immature cochlea will change the peak of response to a specific frequency one octave up, increasing the time of construction of the cochlear filter and, consequently, the latency time. The delayed maturation of high frequencies has also been observed in studies conducted by Trehub in 198716. It is known that the cochlea is still immature in high frequencies after birth, explaining the higher time of latency in newborns. Smurzynsky in 199317, conducted a study of latency in premature babies, full term newborn babies and adults. The results showed higher latency in neonates in comparison to adults for the frequency of 2 to 4 kHz; this reduction of latency depending on age was explained by the maturation changes of the external, middle and inner ears.
Quiñonez, in 19983, conducted a longitudinal study in pre-term newborn babies. They found a significant reduction in latency with post-conceptual age for frequencies of 3 and 4 kHz. This finding suggested incomplete maturation of peripheral hearing in premature babies. They reported that the changes in values of latency could be explained by the changes in acoustic impedance already known18, by the influence of the level of stimulation that reaches the cochlea or the physical changes of the conductive system. During the development of the auditory system, there is the growth of the external acoustic canal (EAC) as well as changes in compliance and resonance. Based on the study by Keefe (1993)18, it is expected to have an increase in acoustic energy transferred to the middle ear after the first 2 months of age, which will result in an increase in acoustic energy that reaches the cochlea. As latency reduces with the increase in level of stimulus 13, the reduction of latency with age is expected. Another variable that could influence the difference in latency between neonates and adults is the change in resonance of the EAC, which reduces from 5-7 kHz in neonates to 3kHz in adults 19. However, all studies show that there is an important change in cochlear micromechanisms owing to maturation, which are important factors for the changes in latency values.
In the present study, we found mean latencies ranging from 5.6ms in 3kHz in males and 4.8 in females to 3.4ms in 6kHz in female and 3.8 for males. Namysloswski et al. in 2001 6 conducted a study of the latency in patients with normal hearing, elderly and exposed to noise. In the group of normal adults, they found latencies of 5.7ms in 3kHz to 4.7ms in 6Khz. They also observed a reduction of latency in elderly subjects and those exposed to noise when compared to normal adults, revealing a degradation of outer hair cells.
When we compared the data of latency values with the variable gender, we found the values were higher for male patients in all tested frequencies except for 6kHz on the left, with significant values for 3kHz on the right. A study in adults with normal hearing 7 also showed higher latency values for DPOAE in males. This difference was significant for frequencies of 720, 2 and 3 kHz on the right ear and only for the frequency of 1282 kHz on the left ear. The difference among genders was reported in a study of latency of adults with normal hearing only in low frequencies 12, 20. This difference was attributed to the difference found in cochlear length of men and women cochlea. Sato et al., 199121, reported that the size of the cochlea in an adult man is 13% longer than in a woman. Kimberley et al., 199320 suggested that when we test the most apical parts of the cochlea (low frequencies), the difference in latency between men and women increases.
The differences found between genders can also be related to the differences in number of OHC between genders, described as higher for female gender 22. In addition, from the 4th to the 8th month of gestation, there is an overproduction of OHC. It is believed that there is a mechanism of production and degeneration that, similarly to cochlea length, is different among the genders. One of these mechanisms can be explained by the innervation of the olivo-cochlear mechanism. Studies showed that cells not innervated by the system degenerate in the cochlear apex 23.
As to the analysis of the latency measure of DPOAE concerning the ear side variable, the results presented allowed the verification that there was no statistically significant difference between the right and left ears of the assessed neonates. In the studied literature we found authors that agreed with this statement 11, 20. However, Abissara (2001)24 found difference between the ears, with greater latency on the left ear in the frequency of 2kHz in subjects with unilateral loss when compared to subjects with normal hearing.
CONCLUSIONBased on the present study, we concluded that there was reduction of latency with increase in tested sound frequency. We observed also a difference in measures of latency between genders and we did not observe difference concerning the ear side.
Based on the analysis of the results presented here it was possible to confirm that the measure of latency of DPOAE can be a useful tool in investigating cochlear function, as well as to better understand the cochlear micromechanisms involved in maturation.
References 1. Norton SJ, Stover LJ. Emissões Otoacústicas - Um novo instrumento clínico. In Tratado de Audiologia Clínica. São Paulo: Editora Manole; 1999. p.444-58.
2. Kemp DT. Stimulated acoustic emissions from the human auditory system - J Acoust Soc Am 1978; 64:1386-91.
3. Quiñonez RE, Crawford MR. Longitudinal Distortion Product Otoacoustic Emissions (DPE) Latency Changes in Preterms Neonates - Acta Otolaryngol (Stocke) 1998; 118:26-31.
4. John MS, Picton TW. Human auditory steady-state responses to amplitude-modulated tones: Phase and Latency measurements. Hearing research 2000; 141:57-79.
5. Von Békésy G. Experiments in Hearing. New York: Mc Graw Hill; 1960.
6. Namylowsky G, Morawsky K, Urbanic P, Trybalska G, LisowaskaG. Latencies of 2 f1-f2 distortion Product otoacoustic emissions measured using a phase-gradient method in yong people, in the eldery and in people exposed to noise. Scand Audiol 2001; 30(52):121-4.
7. Marques VV. Latência das Emissões Otoacústicas - Produto de Distorção em indivíduos audiologicamente normais. Tese de Monografia. Universidade Federal de São Paulo, São Paulo; 2002:2-42.
8. Finitzo T, Albright K, O'neal J. The neoborn with hearing loss detection in the nursery. Pediatrics 1998; 102:1452-60.
9. Kemp DT, Brown A. 1983 - An Integradet view of cochlear mechanical nonlinearieties observable from the ear canal. In: Deboer E, Vierger. Mechanics of Hering Martinus, Nyhoff e Deff:75-82.
10. Pujol R. Maturation of The Cochlea. Series in Audiology 2001; 1:5-10.
11. Maroney CFO, Kemp DT. Distortion product otoacoustic emission delay measurement in human ears. J Acoust Soc Am 1995; 97(6):3721-35.
12. Moulin A, Kemp DT. Multicomponent acoustic distortion product otoacoustic emission phase in humans. II Implications for distortion product otoacoustic emissions generation. J Acoust Soc Am 1996; 100(3):1640-62.
13. Warble J, Collet L, Vachon CB, Croze SC. 2f1-f2 Distortion product otoacoustic amission latency: Changes with frequency and level of primaries. Audiology 1997; 36:72-82.
14. Brown D, Kimberley B, Eggermont J. Cochlear traveling wave delays estimated by DPOE in normal hearing adults and term born neonates. J Otolaryngol 1994; 23(4): 234-7.
15. Zemlin WR. Princípios de anatomia e fisiologia em fonoaudiologia. Porto Alegre: Artmed; 2000.
16. Trehub SE, Schneider BA, Morrongielo BA. Developmental changes in high frequency sensitivity. Audiology 1989; 28:241-9.
17. Smurzynsky J, Jung MD, Lafreniere D. Distortion product and click evocked otoacoustic emissions of preterms and full term infants and adults. Ear Hear 1993; 14:258-74.
18. Keefe DH, Bulen JC. Ear canal impedance and reflection coefficient in human infants and adults. J Acoust Soc Am 1993; 94:2617-38.
19. Keefe DH, Bulen JC, Campbell SL, Burns EM. Pressure transfer function and absorption cross section from the diffuse field to the human infant ear canal. J Acoust Soc Am 1994; 95:355-71.
20. Kimberley BP, Brown DK, Eggemont J. Measuring human cochlear traveling wave delay using distortion product emission phase delay responses. J Acoust Soc Am 1993; 94:1343-50.
21. Sato H, Sando I, Takahashi H. Sexual dimorfism and development of the human cochlea - Acta Otolaryngol (Stockh) 1991; 111:1037-40.
22. Wright A, Davies A, Bredberg G. Hair cell distributions in the normal human cochlea. Acta Otolaryngol 1987; (Suppl) 436:15-24.
23. Zhou SL, Pickles JO. Early hair cell degeneration in the extreme apex of the guinea pig cochlea. Hear Res 1994; 79:147-60.
24. Abissara RGC. Latência das EOA da orelha normal de portadores de perda auditiva neurossensorial unilateral. São Paulo, 2001. Tese de mestrado. Faculdade de Medicina da Universidade São Paulo/ FMUSP.
1 Speech and Hearing Pathologist, Federal University of Sao Paulo EPM, Specialization in Clinical Audiology, University of Sao Paulo - USP.
2 Full Professor, Course of Speech and Hearing Pathology, Medical School, University of Sao Paulo- FMUSP.
Study conducted at the School of Speech and Hearing Pathology, University of Sao Paulo, presented at the Congresso Internacional de Audiologia, in Curitiba, in April 2003.
Address correspondence to: Renata Frasson - Rua Nicolau Gagliardi, 554 apt. 111 Pinheiros SP 05429-010
Tel. (55 11) 3812-3476 / 9801-5730 - E-mail: renatafrasson@uol.com.br
Article submitted on April 23, 2003. Article accepted on August 08, 2003.


