INTRODUCTIONUndifferentiated carcinoma of major salivary glands is a rare pathology, with incidence below 1% among malignant neoplasms of this anatomical structure. It was initially described by Helderman et al.1 (1962) and later correlated with Epstein Barr2-8 virus by many authors.
As of 1992, the World Health Organization (WHO) classified this neoplasm in types of cells - small cells (£ 30 mm) and large cells (>30 mm). However, occasionally, undifferentiated carcinoma is associated with other gland or epidermoid neoplasms (optical microscopy) and to the ultrastructural and antigenic presence of ductal differentiation, epidermoid and neuroendocrine differentiation (electron microscopy and immunohistochemistry), even though these findings are not necessarily markers of prognosis in such neoplasm.
Ki-67 is a no-histonic protein 9, with apparent molecular weight of 345 to 349 Kd, present in all phases of the cell cycle division (except G0), and its name is resultant from its identification in Kiel (Germany) on the 67th dish of tissue culture, and it is an IgG1 of mice produced against a nuclear fraction of cell line L428 of the Hodgkin disease10; it reaches it main expression in phases G2 and M11. Since it is a monoclonal antigen, mean life of one hour 12, chromosome 10 seems to be involves in the protein synthesis of this antigen in the cell cycle 13, 14. Thus, many studies referenced Ki-67 as clinical protector of biological behavior of malignant neoplasms 9, since it diagnoses the synthesis of DNA of the neoplasm, with increased marking of malignant cells with the antigen 11. Another aspect that should be highlighted is the definition of surgical margins based on Ki-67, since it demonstrates a tumor invasion that is sometimes not detected at optical microscopy, being the increase in positivity determinant to define poor prognosis indices.
It is important to point out that positivity of Ki-67 is not exclusively of malignant neoplasm, but it is predominant in comparison to benign lesions of gland tissues 15, 16, being the best marker of malignant transformation, such as for example, in carcinoma former pleomorphic adenoma 17.
Finally, it was emphasized the positive response to Ki-67 in post-radiation gland carcinomas such as recurrence markers 18, being its amount proportional to the level of malignancy of the neoplasm 19, 20.
Therefore, we intend to understand the evolution and prognosis of the undifferentiated carcinoma of the major salivary glands by defining the statistical correlation of the histopathological and immunohistochemical variables, with the proliferation marker Ki-67 by using Ki-67 clone (MIB-1).
MATERIAL AND METHODIn the Departments of Head and Neck Surgery, Otorhinolaryngology and Clinical Pathology (1977 to 2000) we histologically reviewed paraffin blocks of 26 patients with undifferentiated carcinomas of major salivary glands (parotid and submandibular), treated with isolated surgery or associated with radiology. The selected blocks were cut (5m thick), stained with histochemical technique of hematoxylin-eosin (HE) and assessed under optical microscopy according to the WHO criteria (1992), confirming histologically the morphological criteria in only 11 cases.
For the immunohistochemical analysis, we employed the antibody panel of cytokeratin (AE1, AE3, 35B11 and 35B12), Epithelium membrane antigen (EMA), protein S-100, vimentin, common leukocytarian antigen (CLA) and chromogranin, through the method of antigenic recovery and amplification, employing the multivalent antibody (Avitin-Biotin-Peroxidase) in variable dilutions, aiming at confirming epithelial histogenesis of neoplastic cells, together with exclusion of the differential diagnoses, inherent to neoplasia with little level of differentiation (melanoma, non-Hodgkin lymphoma and sarcoma).
As to cell proliferation, we conducted it in two different ways: by mitotic count, made with 10 fields of maximum amplification (CGA), after staining with HE (magnification of 400x), excluding the cases that were not ideally represented. On the second one, we assessed immunohistochemically cell proliferation using monoclonal anti-antigen nuclear antibody Ki-67 (Dalso) clone MIB-1, in dilution of 1/50 (Photo 1). To control the immunohistochemical reactions we used sections of salivary gland fixed in formol and included in paraffin. As positive criterion for Ki-67, we made histology sections of the tonsils with marking of centroblastic lymphocyte in germination centers, with positive nucleoli stained in brown (Photo 2). The quantification of positive responses of clone MIB-1 (Ki-67) was made through count of occurrence of positive nuclei in 500 to 1,000 cells in each case, and there was rigidity of selection of the areas considered positive and negative, excluding the doubtful ones (Photo 3). In ideally represented neoplasms, the lamina was divided into quadrants and followed along so as to provide an equivalent number of cells in the different areas of the fragment.
For statistical purposes, all variables were analyzed using the statistical software GraphPad Instat, version 3.0 and GraphPad Prism, version 3.0. To determine the influence of parametric and non-parametric variables of clinical, demographic and clinical pathologic parameters, we conducted respectively the tests t Student, Fisher, ANOVA and chi-square, with Yates correlation (p < 0.05).
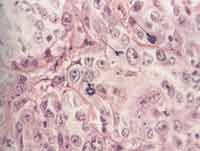
Photo 1. Histolology section of large cell undifferentiated carcinoma of major salivary glands, stained in HE, highlighting the figures of mitosis (400x).
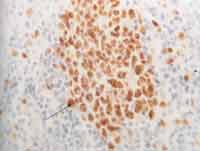
Photo 2. Histology section of tonsil tissue, highlighting the lymphoid cells of germination center (cetroblasts and centrocytes) stained in brown, after immunohistochemistry positive to antibodies Ki-67 clone MIB-1, used as control (400x).
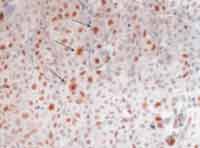
Photo 3. Histology section of large cell undifferentiated carcinoma of major salivary glands, highlighting the neoplastic cells, stained in brown, after immunohistochemical reaction positive to antibody Ki-67 clone MIB-1 (250x).
The results demonstrated there were no statistically significant differences between male gender (63.64%) and female gender (36.36%) resultant from the small size of the sample (p>0.05), even though there had been predominance of Caucasians in 91.0% of the patients (p<0.01) aged 69-11.4 years (Table 1).
As to primary site, the parotid gland predominated significantly in comparison to the submandibular gland (ratio of 10:1), in the proportion of 90.9% to 9.1% (Student's test with p < 0.01). However, there was no statistically significant difference between the right and left sides (54.5% and 36.4%, respectively) analyzed by chi-square test (p > 0.05). As to duration of clinical complaint at diagnosis, it was 4.3 ± 0.6 months (Table 2). As to size of lesion, measuring the tumor in centimeters, there were no statistically significant differences among intervals (Table 3).
As to parameter pT of classification TNM, histology assessment and incidence, even though there were no statistically significant differences through chi-square test (p<0.05), there was numeric predominance of lesions pT2 (2 to 4 cm) (Table 4).
In measuring the mitotic index, in 10 magnification fields (400x), according to the immunohistochemical profile for positive marking of high and low molecular weight cytokeratin, slides were stained with HE, and there were no statistically significant differences according to t Student and ANOVA tests (p>0.05) with immunohistochemical profiles (I, II, II and general) (Table 5).
As to index of immunomarking of Ki-67 (Clone MIB-1) measured in percentage of positivity in 1,000 cells, there were no statistically significant differences in profiles I, II and III of cytokeratins when assessed with t Student and ANOVA tests (Table 6).
As to relation between parameters of mitotic index of cell immunoproliferation of Ki-67 (Clone MIB-1) as a result of immunohistochemical profile of positive markers with high and low molecular weight cytokeratin, there were no statistically significant differences (with t Student test and ANOVA for p>0.05). However, the small sample did not allow statistical assessment of the variables mitotic index/immunoproliferation index of Ki-67 in the groups of Profile X II (n=2), which was possible only in the groups of Profile IPX General, Profile IPX I and Profile IPX III (Table 7).
Table 1. Demographic variables in 11 patients with large cell undifferentiated carcinoma.

* T* t Student test (p<0.01)
Table 2. Distribution of clinical variables in 11 cases of large cell undifferentiated carcinoma of major salivary glands.

*Data were analyzed through Qui 2 test (p< 0.05) concerning predominance of lesion primary topography.
Table 3. Diameter of the lesion versus size of the malignant neoplasm.
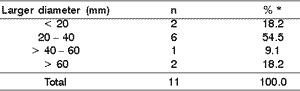
* Data were analyzed with chi-square test (p < 0.05).
Table 4. Relation between pT and malignant neoplasms.
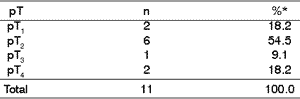
* Data were analyzed with chi-square test (p< 0.05).
Table 5. Mitotic index of undifferentiated carcinomas of major salivary glands concerning immunohistochemical profile of positive markers for cytokeratin of high and low molecular weight.
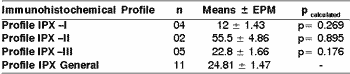
The values were assessed through t Student test (p> 0.05). The results were expressed in mean ± epm of the mitotic index.
Profile IPX I: positivity of high and low molecular weight cytokeratins, in addition to cocktail of cytokeratins AE1AE3.
Profile IPX II: positivity exclusively for low molecular weight cytokeratin.
Profile IPX III: positivity exclusively for high molecular weight cytokeratin.
Profile IPX General: all cases.
Table 6. Index of immunomarker of Ki 67 concerning the immunohistochemical profile of high and low molecular weight cytokeratin in large cell undifferentiated carcinomas of the major salivary glands.
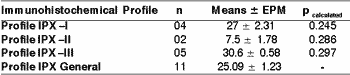
The values were assessed through t Student test (p> 0.05). The results were expressed in mean of immunopositivity of Ki-67 in 1,000 cells (mean ± epm).
Profile IPX I: positivity of high and low molecular weight cytokeratins, in addition to cocktail of cytokeratins AE1AE3.
Profile IPX II: positivity exclusively for low molecular weight cytokeratin.
Profile IPX III: positivity exclusively for high molecular weight cytokeratin.
Profile IPX General: all cases.
Table 7. Distribution by class interval of the marker of cell immunoproliferation Ki 67 concerning immunohistochemical profiles of differentiation.

The values were assessed through chi-square test (p> 0.05).
Profile IPX I: positivity of high and low molecular weight cytokeratins, in addition to cocktail of cytokeratins AE1AE3.
Profile IPX II: positivity exclusively for low molecular weight cytokeratin.
Profile IPX III: positivity exclusively for high molecular weight cytokeratin.
Profile IPX General: all cases.
Undifferentiated carcinomas of major salivary glands owing to their rare occurrence, do not allow positive prognostic classification based on the current morphological concepts, being necessary to associate it with criteria of immunohistochemical profile and cell immunoproliferation marker Ki-67 (Clone MIB-1) for its sub-classification 21, 22. Thus, it is possible to distinguish in these tumors patterns of high and low molecular weight cytokeratins, pointing to bi-directional immunohistochemical differentiation of the type of mucoepidermoid in 4 cases (profile IPX I), ductal/glandular differentiation in 2 cases (Profile IPX II) and epidermoid differentiation in 5 cases (Profile IPX III).
As to marking immunohistochemical profile of high and/or low molecular weight cytokeratins based on the parameters of mitotic index and cell immunoproliferation Ki-67 index, we observed a general mean in the group of 24.81 mitosis in 10/CGA, with standard mean error (EPM) of 1.47 and positivity Ki-67 index of 25.09 ± 1.23 (EPM), according to the profile IPX I (bi-directional marking standard of mucoepidermoid type) of 12 mitoses in 10/CGA and 27 ± 2.31 (EPM) index Ki-67, Profile IPX II (epidermoid differentiation, ductal/glandular) with 22.8 mitoses in 10/CGA and 30.6 ± 0.58 (EPM) of immunomarking index of Ki-67.
Considering undifferentiated neoplasm, Ki-67 is the best marker of cell proliferation that is currently available 23. However, since it does not mark cells G0, upon marking the population growth behavior, it allows the definition of direct correlation with prognosis, that is, it should be added by those non-marked cells (G0). However, whereas we obtain the index of marking of 25.09% in undifferentiated cells, other authors 16 reported lower values for differentiated neoplasms with 14% for epidermoid carcinoma and 13.6% for adenocyst. It allows prediction of the use of the masker as differentiation diagnosis of benign and malignant tumors in patients, when this question has not been answered yet, in situations in which the histology did not provide differentiation based on the methodology normally employed, such as optical macroscopy. It is important to point out that the use of Ki-67 can impact the prognosis of little differentiated or dysplastic neoplasia, in which the level of non-differentiation and dysplasia would be determinant for the prognosis of these neoplasms considering malignancy and behavior throughout the follow up of these patients submitted to isolated surgery or combined with radiotherapy. However, larger samples would be required to allow differentiation of low or high molecular weight cytokeratin patterns, mitotic index as well as immunoproliferation of Ki-67.
CONCLUSIONImmunohistochemical subclassification of positivity to cytokeratins did not present statistically significant differences comparing mitotic index and cell immunoproliferation index (Ki-67) which did not add a variable of predictive importance to the biological behavior of the tumor, considering the histogenic modality of differentiation, requiring further studies with larger samples.
REFERENCES 1. Hilderman WC, Gordon JS, Large HL Jr. Malignant lymphoepitelial leson with carcinomatosous component apparently arising in parotid gland: a malignant counterpart of benign lymphoepithelial lesion? Cancer 1962; 15:606:10.
2. Zur Hausen H, Schullte-Holthausen H, Klein G, Henle W, Henle G, Clifford P, et al. EBV DNA in biopsies of Burkitt´s tumors and anaplastic carcinomas of the nasopharynx. Nature 1970; 228:1056-8.
3. Saemundsen AK, Albeck H, Hansen JPH, Nielsen NH, Anvret M, Henle W et al. Epstein - Barr virus in nasopharyngeal and salivary gland carcinomas of Grenland Eskimoes. Br J Cancer 1982; 46:721-8.
4. Nagao T, Ishida Y, Sugano I, Tajima Y, Matsuzaki O, Hino T et al. Epstein-Barr virus-associated undifferentiated carcinoma with lymphoid stroma of salivary gland in japanese patients. Cancer 1996; 78:695-703.
5. Huang DP, NG HK, Ho YH, Chan KM. Epstein-Barr virus (EBV - associated undifferentiated carcinoma of the parotid gland. Histopathology 1988; 13:509-17.
6. Hamilton-Dutoit SJ, Therkildsen MH, Nielsen NH, Jensen H, Hansen JPH, Pallesen G. Undifferentiated carcinoma of the salivary gland in Greenlandic eskimos: demonstration of Epstein-Barr virus DNA by in situ nucleid acid hybridization. Hum Pathol 1991; 22:811-5.
7. Chen Cl, Ween WN, Chen JY, Hsu HC. Detection of Epstein- Barr virus genome in nasopharyngeal carcinoma by in situ DNA hybridization. Intervirology 1993; 36:91-8.
8. Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma like carcinomas. Am J Clin Pathol 1995; 103:308-15.
9. Gerdes J; Schlueter CLL, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad H. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 1991; 238:867-73.
10. Brownw DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology 1990; 17: 489-503.
11. Sasaki K, Murakami T, Kawasaki M, Takahashi M. The cell cycle associated a change of the Ki-67 nuclear antigen expression. J Cell Phisiol 1987; 133:579-84.
12. Bruno S, Darzynkiewikz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif 1992; 25: 31-40.
13. Garrido MC, Cordell JL, Becker MHG, Key G, Gerdes J, Jones M, Gatter KC, Majon DY. Monoclonal antibody JC1. New reagent for studying cell proliferation. J Clin Pathol 1992; 45:860-5.
14. Key G, Petersen JL, Becker MHG, Duchrow M, Schlüter C, Askaa J, Gerdes J. New antiserum against Ki-67 antigen suitable for double immunostaining of parinf wax sections. J Clin Pathol 1993; 46:1080-4.
15. Giannoni C, El Naggar EK, Ordonez NG, Austin J, Luna MA, Baakis JG. C-erbB-2/neu oncogene and Ki-67 analysis in the assessment of palatal salivary gland neoplasms. Otolaryngol Head Neck Surg 1995; 112:391-8.
16. Murakami M, Ohtani I; Hojo H, Wakasa H. Immunohistochemical evaluation with Ki-67: na application to salivary gland tumours. J Laryngol Otol 1992; 106:35-8.
17. Cooper MP, Braakhuis BJM, de Vries N, Van Dongen GAMS, Nauta JJP, Snow GB. A painel of biomarkers of carcinomarkers of carcinogenesis of the upper aerodigestive tract as potential intermediate andpoints in chemopreventions trials. Cancer 1993; 71:825-30.
18. Valente G, Orecchia R, Gandolfo S, Araudo M, Ragona R, Kerin S, Palestro G. Na Ki-67 immunostaining predict response to radiotherapy in oral squamous cell carcinoma? J Clin Pathol 1994, 47:109-12.
19. Vargas H, Sudilovsky D, Kaplan MJ, Regezi JA, Weidner N. Mixed tumor, polymorphous low grade adenocarcinoma and Adenoid Cystic Carcinoma of the salivary gland. Pathogenic implications and differential diagnose by Ki-67 (MIB1), Bcl1, and S-100 imunohistochemistry. App Imunohistoch 1997, 5:8-16.
20. Lazzaro B, Cleveland D. P53 and Ki-67 antigen expression in small oral biopsy specimens of salivary gland tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89:613-7.
21. Ellis GL, et al. Atlas of Tumor Pathology: Tumors of Salivary Glands, Armed forces Institute of Pathology, Washington, D.C, Third series, 1996.
22. Toyosawa S, Ohnishi A, Ito R. Small cell undifferentiated carcinoma of the submandibular gland: Imunohistochemical evidence of myoepithelial, basal and luminal cell features. Pathology International 1999; 49:887-92.
23. Alves VAF. Manual de Imunohistoquímica. São Paulo: Sociedade Brasileira de Patologia; 1999.
1 Master in Healthcare Sciences, Hospital Heliópolis, Hosphel, Sao Paulo, Brazil.
2 Pathologist, Hospital Heliópolis, Hosphel, Sao Paulo.
3 Coordinator of the Post-Graduation Course, Hospital Heliópolis, Hosphel, Sao Paulo
Study conducted by the Departments of Head and Neck Surgery, Otorhinolaryngology and Clinical Pathology, Hospital Heliópolis, Hosphel, Sao Paulo, Brazil.
Address correspondence to: Prof. Dr. Abrão Rapoport - Rua Iramaia, 136 Jd. Europa 01450-020 Sao Paulo.
Tel (55 11) 289-6229 / 287-4347 - E-mail: cpgcp.hosphel@attglobal.net
Article submitted on February 17, 2003. Article accepted on July 07, 2003.


