INTRODUCTIONCisplatin is a powerful anti-neoplastic drug largely used both in adults and in children to treat advanced cancer cases. Many of the side effects are irreversible and can be clinically monitored, but they can rarely be avoided. Many studies have been conducted in order to find out about drugs that, when associated with cisplatin, can reduce undesirable side effects1-9.
Toxicity of cisplatin has been detected at renal level and central and peripheral nervous system, gastrointestinal toxicity and bone marrow, and similarly to other classes of medications, which cause lesions to Corti's organ, it is considered an ototoxic drug.1,2,4,7,8
Anatomical studies showed that cisplatin causes damage in acute high doses as well as in cumulative doses, especially impairing outer hair cells, causing an initial lesion in the basal turn cells of the cochlea. Some authors suggested the occurrence of a block in the transduction of calcium channels of such cells, others demonstrated lesions to outer hair cells, support cells and stria vascularis, as well as spiral ganglion 10-17.
Cisplatin causes bilateral and irreversible hearing loss in human beings, with associated tinnitus, affecting the high frequency range (4000 Hz to 8000 Hz).1,2,4,8,18
The study of the effects of cochlear toxicity has been directed by electrophysiological abnormalities detected by Auditory Brainstem Audiometry and endocochlear potential.2,4,5,15,16,19-21
Otoacoustic emissions detected by Kemp (1971) in the external auditory canal of human beings measure the feedback of biomechanical energy in contracting outer hair cells, which amplify the peak of the traveling wave at the basilar membrane and have clinical application in the assessment of auditory integrity 16, 21-25.
Since cisplatin initially acts by causing damage to the outer hair cells, otoacoustic emissions would be a simple and quick assessment method of the damage, including monitoring of patients submitted to drugs considered to be ototoxic 16, 21-26.
Studies have shown the possibility of an antioxidant mechanism for ototoxicity and nephrotoxicity generated by cisplatin, since the toxicity clearance routes in tissues are similar. Authors have shown that the levels of glutatione and antioxidant enzyme activity such as dismutase superoxide, catalase, GSH peroxidase and GSH reductase are reduced in such tissues, leading to lipid peroxidation and, consequently, cell toxicity10, 27-34.
Many drugs have been tested in order to provide otoprotection, such as sodium thiosulfate, diethyldithiocarbamate, ACTH and by-products, 4-methylthiobenzoic acid, lipoic acid, glutatione and esters, methionine, procaine, alpha-melanocytic stimulating hormone (melatonin), antioxidants such as ginkgo biloba and fosfomycin and sulfur compounds.3,5,6,9,35-47
A recent master dissertation at Medical School of Ribeirao Preto showed low doses of gentamicin leading to a structural protection effect or outer hair cells, which maintained their normal characteristics at scanning electron microscopy when later applied high doses of the drug, suggesting, therefore, that there is a self-defense mechanism - intracochlear adaptation to insults induced by ototoxic drugs 48-51.
The purpose of the present study was to check whether there is an otoprotective effect of drugs that are used in clinical practice to other ends, such as ginkgo biloba, to the cochlear damage caused by cisplatin, using doses of cisplatin that are known to be ototoxic, harmful to the outer hair cells and assessing anatomical abnormalities through electron microscopy and functional distortion product otoacoustic emissions.
MATERIAL AND METHODAlbino guinea pigs were selected as experimental animals since they are easy to handle, to dissect the cochlear region and to manipulate it, easy for infusion routes of anesthetic drugs and experimenting drugs, via peritoneal region or intramuscular region. In addition, compared to rats, for example, it is more sensitive to cisplatin effects and appropriate care can be provided, according to the experimental animal care rules11, 12, 14, 37, 52-54.
Animals were divided into 4 study groups:
Group 1: 4 animals - 8 cochleae - cisplatin 8.0 mg/Kg/day (IP) for 8 days.
Group 2: 6 animals - 12 cochleae - Ginkgo biloba 100 mg/Kg PO and 90 minutes after, cisplatin 8.0 mg/Kg/day (via intraperitoneal) for 8 days.
Group 3: 6 animals - 12 cochleae - Ginkgo biloba 100 mg/Kg oral route for 10 days and after Ginkgo biloba 100 mg/Kg PO and 90 minutes after, cisplatin 8.0 mg/Kg/day (via intraperitoneal) for 8 days.
Group 4: 3 animals - 6 cochleae - Ginkgo biloba 100 mg/Kg PO for 8 days.
Used drugs:
1. Cisplatin (8.0 mg/ml) - Pharmaceutical company - Eurofarma
8.0 mg/day via intraperitoneal
2. Xilazin (Coopazine®, Rompun®) 2g/100ml (6.5mg/Kg)
3. Ketamin Chloridrate (Ketamin®) 50 mg/ml (65mg/Kg)
4. Extract of ginkgo biloba (EGB 761) - Pharmaceutical company - ByK Química 40 mg/ml (100 mg/Kg/day)
1. Distortion product otoacoustic emissions
EQUIPMENT: ILO 92 CAE System Otodynamics LTD.
2. Scanning electron microscopy
Microscope Jeol - JSM 5200.
Distortion product otoacoustic emissions
Guinea pigs were anesthetized with CoopazineÒ and Ketamin® to be submitted to the test. Before the test, we submitted them to manual otoscopy for the assessment of external auditory canal and tympanic membrane, and we excluded the guinea pigs that presented signs of otitis and cerumen that could not be removed.
The distortion product otoacoustic emissions test was conducted before treatment and minutes before the animals were sacrificed following the relation 2F1 - F2 with F1:F2 = 1.22 ratio, resolution 1 point per octave.
Scanning electron microscopy 11,33,49,55
Guinea pigs were sacrificed within the defined schedule after the administration of intraperitoneal drugs and then they were sacrificed after anesthesia with ether, being decapitated, and their cochleae were removed from the bulla.
Microscopic dissection of the cochlear followed perfusion with fixation solution with glutaraldehyde at 3% and 4º Celsius, maintained in the solution for 24 hours for fixation. The next steps were conducted in the Laboratory of Electron Microscopy, Department of Morphology FMRP-USP.
Through the round window, and for fixation purpose, we injected a solution of glutaraldehyde at 3% in phosphate buffer 0.1 M, pH = 7.4, for 4 hours at 4º Celsius, washed 3 times for 5 minutes with the same buffer solution and then they were fixated with osmium tetroxide 1% for 2 hours at 4o Celsius and submitted to dehydration at room temperature in a increasing battery of ethanol (50%, 70%, 90% and 95% - once for 10 minutes at each concentration) and absolute ethanol 3 times for 15 minutes. When the dehydration was finished, we proceeded to drying by using the method of CO2 critical point in which the material was released from water. After fixation in the appropriate holder, the material was recovered in a vacuum chamber with gold vapors and examined under scanning electron microscopy.
We used the electron microscope JEOL SCANNING MICROSCOPE - JSM 5200.
The results obtained with scanning electron microscopy, after being photographed, were analyzed using cochleogram (count of outer hair cell number on the basal turn of cochlea in a specific photographic field) and statistically assessed by t-test, using the statistical program Primer of Biostatistics (Stanton A. Glantz).
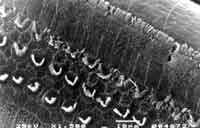
Figure 1. Scanning electron microscopy photo of one of the guinea pigs treated with Cisplatin 1.5 mg/Kg/day for 18 days - post-treatment (on sacrifice day).
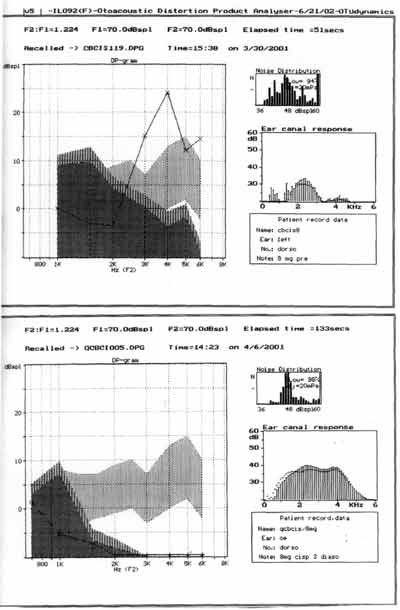
Figure 2. Distortion product otoacoustic emissions of one of the guinea pigs treated with cisplatin 8.0 mg/Kg/day for 8 days -pre and post-treatment (on sacrifice day).
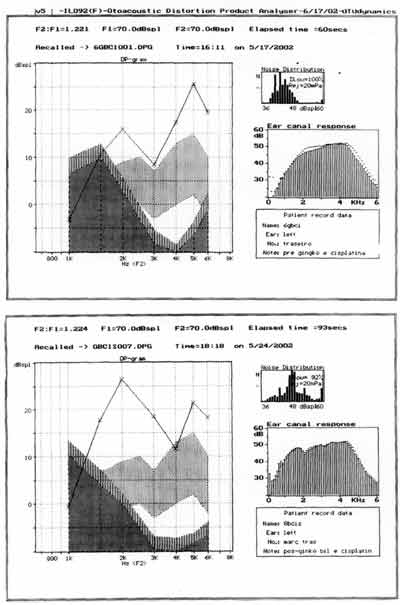
Figure 3. Distortion product otoacoustic emissions of one of the guinea pigs treated with ginkgo biloba 100 mg/Kg/day 90 minutes before cisplatin 8.0 mg/Kg/day for 8 days - pre and post-treatment (on sacrifice day).
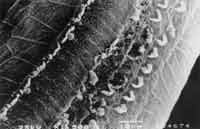
Figure 4. Surface electron microscopy of the cochlea of guinea pig treated with cisplatin 8.0 mg/Kg/day for 8 days. Affections of outer hair cells, 3 rows of hairs with derangement of remaining hairs and hair distortion in inner hair cells (basal turn).
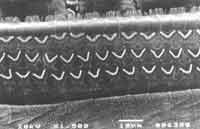
Figure 5. Surface electron microscopy of the cochlea of a guinea pig treated with ginkgo biloba extract 100 mg/ day and cisplatin 8.0 mg/Kg/day for 8 days. Normal pattern on cochlea basal turn with cilia organization and normal pattern of inner hair cells cilia.
As to the dosage of cisplatin used, in previous studies (unpublished data), we had used the dosage of 1.5mk/kg/day for 18 days and we did not observe anatomical abnormalities in the outer hair cells of the basal turn of albino guinea pigs treated with cisplatin, and there had been no abnormalities of distortion product otoacoustic emissions before and after treatment (Figure 1).
The assessment of the functional status of outer hair cells studied by distortion product otoacoustic emissions showed reduction in the amplitude of tested frequencies as of 2kHz, being the responses identified in the noise area of the group treated with cisplatin 8.0 mg/Kg/day for 7 days (Figure 2).
As to otoacoustic emissions of group 2, treated with ginkgo biloba before cisplatin, results are presented by Figure 3. Distortion product otoacoustic emissions assess the functional status of outer hair cells at the cochlear basal turn, but the assessment is quantitative. We considered the distortion product otoacoustic emissions as present or absent. In groups 2 and 3 treated with ginkgo biloba, distortion product otoacoustic emissions were present in all tested ears.
Comparing groups 2 and 3, we observed that the tracing of distortion product otoacoustic emissions were absent in all ears of the group (n=8) with statistically significant differences (Fisher exact test: p = 0.003).
As to anatomical assessment, in the group treated with isolated cisplatin (8.0mg/kg/day) there was damage and absence of cilia in the three rows of outer hair cells at the level of the basal turn, followed by turns 2 and 3. The most evident abnormalities were in the basal turn, but we also observed ciliary distortion with standard derangement in V (or W) pattern with bent cilia or partial absence of one of the arms of the V (Figure 4). At the level of the inner hair cells we could also observe ciliary abnormalities, with cilia present but in a deranged format.
In the group previously treated with ginkgo biloba (group 2), we detected the presence of all outer hair cells in the basal turn without cilia distortion both in outer and inner hair cells. In turns 2 and 3, there were few absences of cilia in rows 1 and 2 of outer hair cells, without ciliary distortion and abnormalities in inner hair cells (Figure 5).
We selected a fourth group with 3 guinea pigs in which we used only ginkgo biloba extract in the dosage of 100mg/Kg/day for 8 days and we observed that otoacoustic emissions were present in the period and surface electron microscopy showed the presence of outer hair cells in all cochlear turns.
As to statistical analysis of data collected through cochleogram of basal turn of studied cochleae between groups 2 and 3, there were no statistically significant differences (t-test: p= 0.591).
As to cochleogram of groups 1 and 2 and groups 1 and 3, we analyzed them statistically and we detected statistically significant difference (t-test: p < 0.001).
DISCUSSIONWe know that cisplatin leads to ototoxicity by damaging outer hair cells and that otoacoustic emissions assess the outer hair cell contraction status, being the simplest and most representative method for assessing the functional status of these cells, thus distortion product otoacoustic emissions were used in this study.
Up to recently, most studies about ototoxicity by cisplatin had assessed functional abnormalities of the cochlea by means of action and summation potential and cochlear microphonic reaction or by auditory brainstem response. Sokalingam et al. (2000) noticed that the registration of otoacoustic emissions, transient or distortion product, is a sensitive method to assess the functional status of outer hair cells, being that the doses 10 to 12 mg/Kg/day in 3 days already cause affection of distortion product otoacoustic emissions and outer hair cell damage, decreasing distortion product amplitude more than transient otoacoustic emissions 16, 21, 23-26, 54.
As to the dose of cisplatin selected to the study, there are many studies in the literature that refer to dosage, route of administration and ideal duration of use for the study of ototoxicity effects of cisplatin. Cardinal et al. (2000) showed that in doses of 1.5 to 2.0 mg/Kg /day intraperitoneal cisplatin in albino guinea pigs there is 60% to 65% cell loss in 8 days, shown in the basal turn of the cochlea11, 17, 18, 46, 51.
In doses of 8.0 to 15.0 mg/Kg of intraperitoneal administration, single dose, there are affections of distortion product otoacoustic emissions and auditory brainstem responses 11, 54.
We selected the dose of 8.0 mg/Kg/day for 8 days because we expected to find damage of over 65% of the outer hair cells of the basal turn, with evident affections in distortion product otoacoustic emissions and electron microscopy 11.
In the studied group in which we used 80mg/Kg/day 3 to 7 days, we detected cell damage to the three rows of outer hair cells and some affections of the inner hair cell rows.
As to the mechanisms involved in cisplatin ototoxicity, different studies have tried to differentiate such mechanisms so that we could act more precisely and efficiently with the use of otoprotective agents.
Ravi et al. (1995), upon studying the effects of cisplatin over the antioxidant mechanisms of the cochlea through glutatione, glutatione oxide and malonyldialdehyde levels, including the activity of antioxidant enzymes, such as dismutase superoxide, catalase, glutatione peroxidase and glutatione reductase, noticed that the activity of dismutase superoxide and catalase and the level of malonyldialdehyde were significantly increased and the activities of glutatione peroxidase and glutatione reductase were significantly reduced in the cochlea under the action of cisplatin 27.
Recent studies showed that such mechanism could explain the ototoxic effects of cisplatin 30, 31, 40, 50.
Smoorenburg et al. (1999) showed that the abnormalities of antioxidant systems of outer hair cells are important and that different otoprotective agents tested to the present act to prevent the interaction between platinum and dismutase superoxide, preventing the formation of free radicals, deviating the footplate from its toxic site and can interact directly with cisplatin; however, these two last mechanisms can interfere in the anti-neoplastic power of cisplatin 32, 50.
Huang et al. (2000) showed cochlear damage with outer hair cell apoptosis when submitted to oxidative stress. Cisplatin leads to the formation of reactive oxygen forms and intracellular free radicals that interact with phospholids of the outer hair cell membrane, leading to peroxidation aldehyde lipid (4-hydroxinmonenal) that is a known mediator of apoptosis for outer hair cells and auditory neurons. Thus, it is proposed that otoprotection would act in the antioxidant mechanism, which can act in three basic points: 1) prevention of reactive oxygen formation; 2) neutralization of toxic products of lipid peroxidation; and 3) blockage of substances that damage the membrane of sensorial cells, blocking the apoptosis mediators 33, 34.
Different substances have been tested in order to otoprotect from cisplatin, such as diethylcarbamate, 4-methylthiobenzoic acid, ebselen, melatonin, lipoic acid9,40,42,43,45, acetyl cysteine 38, fosfomycin47, neurotrofines39, analogs of ACTH.20,41,44
Many of such substances have side effects in vivo and nothing is known about their action to block the anti-neoplastic effects of cisplatin.
Fukaya et al. (1999) tested the effects of dry extract of ginkgo biloba (egb 761) over ototoxicity of cisplatin as being a possible otoprotective agent. In this study the authors used albino rats, the cisplatin dosage used was 1.5mg/Kg/day for 10 days, with physiological measure of action potential and histology study. They observed that ginkgo biloba protected the outer hair cells 46.
Ginkgo biloba presents antioxidant properties, reduces lipid peroxidation, increases levels of catalase, dismutase superoxide and glutatione. In plasma, they also form complexes of phospholipids, increasing its antioxidant activity. At intracellular level, it acts at the mitochondriae, oxidative phospholiration of mitochondrial RNA and Golgi complex level, helping the DNA repair mechanism and improving cell antioxidant status, increasing the levels of intracellular glutatione and reducing the incorporation of 3H-thimidine 56-62.
Our intention was to try to confirm such findings, using as experimental animal the albino guinea pig, which has proved to be very sensitive to the toxic effects of cisplatin, when compared to albino rats, and to use high doses of cisplatin, which are definitely harmful to outer hair cells16, 46.
Similarly to Fukaya et al., we observed that there is a protective effect of ginkgo biloba for outer hair cells, and did not detect distortion product affection 8 days after the use of cisplatin (80mg/Kg/day) when associated with ginkgo biloba; we did not find damage to outer hair cells at scanning electron microscopy in the cochlear basal turns, in turns 2 and 3 we found absence of 1 or 3 cells in half of the analyzed cochleae.
Considering the ototoxicity mechanisms of cisplatin, related to the mechanisms of cell antioxidation and formation of free radicals, and that ginkgo biloba extract leads to reduction of lipid peroxidation, with cleaning of superoxides, preventing the formation of free radicals, in addition to being a medication that is largely used in human beings with few side effects and evidence of otoprotection against cisplatin, there is the possibility that the concomitant use of ginkgo biloba and cisplatin can minimize the ototoxic effects, both in adults and in children.
CONCLUSIONS1. Dry ginkgo biloba extract (egb 761) has a protective effect on outer hair cells, in cochlea of albino guinea pigs against the aggression of cisplatin, probably owing to its modulating effect on the anti-oxidant defense system of such cells, preventing the formation of cell free radicals or favoring the elimination by the cell exposed to oxidative stress, as it is believed to be the action mechanism of ginkgo biloba.
2. Since it is a largely used drug in clinical practice and since there are no significant side effects, as well as no descriptions of interactions with other drugs, ginkgo biloba can be studied in human beings as a potential otoprotective agent against the effects of ototoxic drugs such as cisplatin.
REFERENCES]
1. Laurell G, Engström B, Bagger-Sjöback D. Ototoxicity of Cisplatin. Int J Androl 1987;10:359-62.
2. Powis GD, Hacker MP. The Toxicity of Anticancer Drugs. New York: Pergamon Press; 1991. 82-105.
3. Rybak LP, Radhika R, Somani SM. Mechanism of Protection by Diethyldithiocarbamate against Cisplatin Ototoxicity: Antioxidant System. Fundamental and Apllied Toxicology. 1995;26:293-300.
4. Stadnicki SW, Gleischman RW, Schaaeppi U. Ototoxicity of Cisdichlorodiammine Platinum (II) (NSC-119875): Hearing loss and Other Toxic Effects in Rhesus Monkeys. Can Chemother Ver 1975;59:467-80.
5. Stengs CHM, Klis SFL, Huizing EH, Smoorenburg GF. Protective Effects of a Neurothophic ACTH (4-9) Analog on Cisplatin Ototoxicity in Relation to the Cisplatin Dose: An Electrocochleographic Study in Albino Guine Pigs. Hearing Res 1998;124:108-17.
6. Tognela S. Pharmacological Interventions To Reduce Platinum-induced Toxicity. Can Trat Ver 1990;17:139-42.
7. Towflight J, Strauss M, Lord S. Cisplatin Ototoxicity: Clinical Experience and Temporal Bone Hystopatology. Laryngoscope 1983;93:1554-9.
8. Wright CG, Schaeffer SD. Inner Ear Hystophatology in patients Treated With Cisplatin. Laringoscope 1982;92:1408-13.
9. Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol 2000;21(4):513-20.
10. Barron SE, Daihneault EA. Effect of Cisplatin on Hair Cell Morphology and Lateral Wall Na-K-ATP ase Activity. Hear Res 1987;26:131-7.
11. Cardinaal RM, Groot JCMJ, Huizing EH, Veldman JE, Smoorenburg GF. Dose-Dependent Effect of 8-Day Cisplatin Administration Upon the Morphology of the Albino Guinea Pig Cochlea. Hearing Res 2000;144:135-46.
12. Janning MH, Withworth CA, Rybak LP. Experimental Model of Cisplatin Ototoxicity in Chinchilas. Otolaryngol Head Neck Surg 1998;119(6):574-80.
13. Kohn SM, Fradis J, Zidan L, Podoshin E, Robinson & I Nir. Cisplatin Ototoxicity in Guinea Pigs with Special Reference to Toxic Effects in the Stria Vascularis. Laringoscope 1988;8;885-71.
14. Laurell G, Engström B. The Ototoxic Effect of Cisplatin in Guinea Pigs in Relation to Dosage. Hear Res 1989;38:27-34.
15. Stengs CHM, Klis SFL, Huizing EH, Smoorenburg GF. Cisplatin Ototoxicity. An Electrophysiological Dose-Effect Study in Albino Guinea Pigs. Hearin Res 1998;124:99-107.
16. Sockalingam R, Freeman S, Cherny TL, Sohmer H. Effect of high-dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. Am J Otol 2000; 21(4):521-7.
17. Stengs CH, Klis SF, Huizing EH, Smoorenburg GF. Cisplatin ototoxicity. An electrophysiological dose-effect study in albino guinea pigs. Hear Res 1998;124(1-2):99-107.
18. Saito T, Aran JM. Comparative ototoxicity of cisplatin during acute and chronic treatment. ORL J Otorhinolaryngol Relat Spec 1994;56(6):315-20.
19. Shaw NA. The Auditory Evoked Potential in the Rat - a Review. Prog Neurobiol 1988;31:19-45.
20. Stengs CH, Klis SF, Huizing EH, Smoorenburg GF. Protective effects of a neurotrophic ACTH(4-9) analog on cisplatin ototoxicity in relation to the cisplatin dose: an electrocochleographic study in albino guinea pigs. Hear Res 1998;124(1-2):108-17.
21. Sie KC, Norton SJ. Changes in otoacoustic emissions and auditory brain stem response after cis-platinum exposure in gerbils. Otolaryngol Head Neck Surg 1997;116(6 Pt 1):585-92.
22. Harel N, Kakigi. A, Hirakawa H, Mount RJ, Harrison RV. The Effects of Anesthesia on Otoacoustic Emissions. Hearing Res 1997;110:25-33.
23. Cevette MJ, Drew D, Webb TM, Marion MS. Cisplatin ototoxicity increased DPOAE amplitudes and magnesium deficiency. Distortion product otoacoustic emissions. J Am Acad Audiol 2000;11(6):323-9.
24. Allen GC, Tiu C, Koike K, Ritchey AK, Kurs-Lasky M, Wax MK. Transient-evoked otoacoustic emissions in children after cisplatin chemotherapy. Otolaryngol Head Neck Surg 1998;118(5):584-8.
25. Ozturan O, Jerger J, Lew H, Lynch GR. Monitoring of cisplatin ototoxicity by distortion-product otoacoustic emissions. Auris Nasus Larynx 1996;23:147-51.
26. Ress BD, Sridhar KS, Balkany TJ, Waxman GM, Stagner BB, Lonsbury-Martin BL. Effects of cis-platinum chemotherapy on otoacoustic emissions: the development of an objective screening protocol. Third place Resident Clinical Science Award 1998. Otolaryngol Head Neck Surg 1999;121(6):693-701.
27. Ravi R, Somani SM, Rybak LP. Mechanism of Cisplatin Ototoxicity: Antioxidant System. Pharmacology & Toxicology. 1995;76:386-94.
28. McAlpine D, Johnstone BM. The Ototoxic Mechanism of Cisplatin. Hear Res 1990;47:191-203.
29. Miettinen S, Laurell G, Andersoson A, Johanson R, Laukikainen E. Blood Flow-Independent Accumulation of Cisplatin in the Guinea Pig Cochea. Acta Otolaryngol (Stockh) 1997;117:55-60.
30. Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol 2001;1174(1):27-34.
31. Watanabe KI, Hess A, Bloch W, Michel O. Nitric oxide synthase inhibitor suppresses the ototoxic side effect of cisplatin in guinea pigs. Anticancer Drugs 2000;11(5):401-6.
32. Evans P, Halliwell B. Free radicals and hearing. Cause consequence and criteria. Ann N Y Acad Sci 1999;28;884:19-40.
33. Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, Lefebvre PP, Malgrange B, Kopke R, Moonen G, Van De Water TR. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci 2000;18(2-3):259-70.
34. Maheswari KU, Ramachandran T, Rajaji D. Interaction of cisplatin with planar model membranes - dose dependent change in electrical characteristics. Biochim Biophys Acta 2000;15;1463(2):230-40.
35. Rybak LP, Whitworth MA, Somani S. Application of Antioxidants and Other Agents to Prevent Cisplatin Ototoxicity. The Laringoscope Nov 1999;109:1740-4.
36. Cardinaal RM, Groot JCMJ, Huizing EH, Veldman JE, Smoorenburg GF. Histological Effects of Co-administration of an ACTH(4-9) Analog ORG 2766 on Cisplatin Ototoxicity in the Albino Guinea Pig. Hearing Res 2000;144:157-67.
37. Laurell G, Engström B. The Combined Effect of Cisplatin and Furosemida on Hearing Function in Guinea Pigs. Hearing Res 1989;38:19-26.
38. Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 2001;111(7):1147-50.
39. Gao WQ. Role of neurotrophins and lectins in prevention of ototoxicity. Ann N Y Acad Sci 1999;28:312-27.
40. Rybak LP, Somani S. Ototoxicity. Amelioration by protective agents. Ann N Y Acad Sci. 1999;28:143-51.
41. Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Histological effects of co-administration of an ACTH((4-9)) analogue ORG 2766 on cisplatin ototoxicity in the albino guinea pig.Hear Res 2000;144(1-2):157-67.
42. Kamimura T, Whitworth CA, Rybak LP. Effect of 4-methylthiobenzoic acid on cisplatin-induced ototoxicity in the rat. Hear Res 1999;131(1-2):117-27.
43. Rybak LP, Husain K, Whitworth C, Somani SM. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system.Toxicol Sci 1999;47(2):195-202.
44. Heijmen PS, Klis SF, De Groot JC, Smoorenburg GF. Cisplatin ototoxicity and the possibly protective effect of alpha-melanocyte stimulating hormone. Hear Res 1999;128(1-2):27-39.
45. Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 2000;28(2):73-80.
46. Fukaya H, Kanno H. Experimental studies of the protective effect of ginkgo biloba extract (GBE) on cisplatin-induced toxicity in rats. Nippon Jibiinkoka Gakkai Kaiho1999;102(7):907-17.
47. Jordan JA, Schwade ND, Truelson JM. Fosfomycin does not inhibit the tumoricidal efficacy of cisplatinum. Laryngoscope 1999;109(8):1259-62.
48. Canedo DJM. Resistência a Ação Ototóxica de Antibióticos Aminoglicosídeos. Tese de Mestrado. Faculdade de Medicina de Ribeirão Preto - USP. 1999.
49. Cardinaal RM, Groot JCMJ, Huizing EH, Veldman JE, Smoorenburg GF. Cisplatin-Induced Ototoxicity: Morphological Evidence of Spontaneous Outer Harir Cll Recovery in Albino Guinea Pigs? Hearing Res 2000;144147-156.
50. Smoorenburg GF, De Groot JC, Hamers FP, Klis SF. Protection and spontaneous recovery from cisplatin-induced hearing loss. Ann N Y Acad Sci 1999;28;884:192-210.
51. Klis SF, O'Leary SJ, Hamers FP, De Groot JC, Smoorenburg GF. Reversible cisplatin ototoxicity in the albino guinea pig. Neuroreport 2000;11(3):623-6.
52. Ekborn A, Laurell G, Anderson A, Wallim I, Eksborg S, Ehrsson H. Cisplatin-Induced Hearing Loss: influence of the Mode of Drug Administration in the Guinea Pig. Hearing Res 2000;140:38-34.
53. Taudy M, Syka J, Popelár J, Úlehlová L, Carboplatin and Cisplatin Ototoxicity in Guinea Pigs. Audiology 1992;31:293-299.
54. Hatzopoulos S, Di Stefano M, Albertin A, Martini A. Evaluation of cisplatin ototoxicity in a rat animal model. Ann N Y Acad Sci 1999;28:211-25.
55. Saito T, Manabe Y, Honda N, Yamada T, Yamamoto T, Saito H. Semiquantitative analysis by scanning electron microscopy of cochlear hair cell damage by ototoxic drugs. Scanning Microsc 1995;9(1):271-80; discussion 280-1.
56. Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res 2001;15(5):449-51.
57. Sasaki K, Hatta S, Wada K, Ueda N, Yoshimura T, Endo T, Sakata M, Tanaka T, Haga M. Effects of extract of Ginkgo biloba leaves and its constituents on carcinogen-metabolizing enzyme activities and glutathione levels in mouse liver. Life Sci 2002;22;70(14):1657-67.
58. Carini M, Aldini G, Rossoni G, Morazzoni P, Facino RM. Complexation of Ginkgo biloba extract with phosphatidylcholine improves cardioprotective activity and increases the plasma antioxidant capacity in the rat. Planta Med 2001;67(4):326-30.
59. Siddique MS, Eddeb F, Mantle D, Mendelow AD. Extracts of Ginkgo biloba and Panax ginseng protect brain proteins from free radical induced oxidative damage in vitro. Acta Neurochir Suppl 2000;76:87-90.
60. Louajri A, Harraga S, Godot V, Toubin G, Kantelip JP, Magnin P. The effect of ginkgo biloba extract on free radical production in hypoxic rats. Biol Pharm Bull 2001;24(6):710-2.
61. Gohil K, Moy RK, Farzin S, Maguire JJ, Packer L. mRNA expression profile of a human cancer cell line in response to Ginkgo biloba extract: induction of antioxidant response and the Golgi system. Free Radic Res 2000;33(6):831-49.
62. Fosslien E. Mitochondrial medicine-molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci 2001;31(1):25-67. Review.
1 Assistant physician.
2 Faculty Professor.
3 Laboratory technicians.
Otorhinolaryngology, Department of Ophthalmology, Otorhinolaryngology and Head and
Neck Surgery, Medical School, Ribeirão Preto - University of Sao Paulo.
Medical School, Ribeirão Preto - University of Sao Paulo.
Address correspondence to: Prof. Dr. José Antônio A.de Oliveira - Area de Otorrinolaringologia do Departamento de Oftalmologia,
Otorrinolaringologia e Cirurgia de Cabeça e Pescoço da Faculdade de Medicina de Ribeirão Preto - Universidade de São Paulo - Campus USP.
Avenida Bandeirantes, 3900 Ribeirão Preto SP 14049-900
Tel (55 16) 602-2863 - Fax (55 16) 602-2860
Article submitted on May 13, 2003. Article accepted on June 05, 2003.


