INTRODUCTIONInfections of the upper respiratory tract, rhinosinusitis and allergic rhinitis are part of the most common causes of primary medical care1,2. Decreased school performance, work productivity, lost days and high treatment costs related to health care and prescribed medications are some of the important social-economic impact of these diseases. Therefore, several adjuvant therapies have been used with the purpose to effectively treat nasosinusal diseases and to cut costs. Some of these therapies include nasal irrigation, humidification, and hyperthermia 3,4.
The benefits of nasal irrigation as adjuvant therapy for nasosinusal diseases have been known since the 19th century. Consistent success obtained at that time in patients with infectious diseases such as syphilis and tuberculosis prolonged its use during the 20th century5. Since then, doctors have been prescribing nasal irrigation as adjuvant therapy for the treatment of acute and chronic 2,6,7viral and bacterial rinosinusitis1-4,6, allergic rhinitis 4,8,9, atrophic and Ozena rhinitis 8-10, drug-induced rhinitis8, and other nasosinusal diseases9,11 as well as in the postoperative care of surgeries of the nose and paranasal sinuses 2,4,12.
The advantages of nasal irrigation include immediate clearance of purulent secretion and mucus of nasopharynx, and possible clearance of the septal ostium, restoration of airway patency, reduction of mucosa edema, material sampling for viral and bacterial diagnostic investigation and probable reduction in the use of antibiotics. In addition to improving symptoms of nose diseases, it still facilitates the application and access of topical nose drugs 13 increasing their efficacy9. The disadvantages of this procedure include reluctance from parents or from patients against the use of nasal lavage, and the small risk of aspirating fluid containing contaminated nasal secretion or of introducing such fluid in the middle ear through the auditory tube 13. In postoperative care of nasal surgeries, nasal irrigation results in major benefits, since it removes crusts, secretions and debris14.
Benefits and efficacy of nasal irrigation have been demonstrated in several clinical trials, however most of these studies are based on the saccharine test as a form to evaluate the efficacy of the solution under investigation11,14-16. We could not find any evidences about local effect of this procedure or little is known about the effects of the use of different solutions in nasal lavage9. Nonetheless, there are some controversies related to hydroelectrolytic components of solutions to be applied, and about the proper concentration of these solutions.
Additional studies to assess local effects of using these hydroelectrolytic solutions for nasal irrigation are required to establish the proper components and the concentration of each electrolyte to be used without modifying the morphological and functional characteristics of nasal and septal epithelium.
The objective of this study was to determine whether tested hydrolytic solutions are adequate to be used in nasal irrigation or not based on histological findings of nasal mucosa of rats that have undergone this procedure.
MATERIAL AND METHODSWistar rats (120) were selected, weighting approximately 300g and 12 weeks old. Animals were treated and kept under the same conditions in the Central Animal Research Center of the Federal University of Sao Paulo - Escola Paulista de Medicina (UNIFESP-EPM), in the Development Center of Experimental Models for Medical and Biology Sciences(CEDEME).
Animals were equally divided into four groups: A, B, C and D. Group A was treated with solution 1 (0.9% Sodium Chloride 0.9%); Group B was treated with solution 2 (0.6% Sodium Chloride, 0.03% Potassium Chloride, and 5% Glucose); Group C was treated with solution 3 (1.2% Sodium Chloride, 0.03% Potassium Chloride and 1% Glucose). The animals of Group D were used as controls and were kept under the same conditions as the animals in the other groups, but did not receive any kind of nasal treatment. Each group was then equally subdivided into I and II, according to the exposure time in the study as follows: subgroup I - exposed for one week, and subgroup II - exposed to the treatment for 4 weeks.
The 0.1 ml (two drops) volume was infused twice a day in the left nostril of the animals using a syringe, one in the morning and the other in the evening without any intervals or treatment interruptions.
The right nostril was initially preserved for control analysis (in addition to the control-group previously assigned as group D). Nevertheless, after observing frequent reflux of the solutions applied contralaterally in the nostril ,the investigators decided to keep the analysis of the results only of the left nostril.
Immediately after the end of each exposure interval, animals of each specific group were sacrificed and subject to median rhinotomy with removal of nasal septum and its mucosa, demarked to the left nasal fossa for histopathological analysis. The fragments of collected tissues were stained in 10% Formaldehyde solution and sent to the Department of Pathology of the Federal University of Sao Paulo, for macroscopic analysis with two transversal sections of the middle third of the nasal septum of each sample. Then, sections were subjected to sequential histological processing. The staining substances used were H&E. The evaluation of the histological sections collected was performed with optical microscopy Olympus BX40 (nº 8f07805) microscope model. The analysis was blind and always performed by the same investigator (Figure 1).
The two histological aspects studied were based upon epithelial abnormalities found in the studies involving nasal mucosa of rats exposed to different local stimuli 17-20. The evaluation of the findings included qualitative aspects such as inflammatory infiltrate and proliferation of intraepithelial glands (Figures 2 and 3).
RESULTSThe use of a log-linear model with linear association was required to study the relationship among the result of the inflammatory infiltrate or intraepithelial gland proliferation, the groups and exposure time.
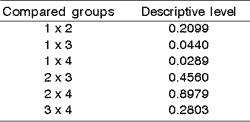
The inferential analysis of inflammatory infiltrate showed a difference trend among the groups (p= 0.1098). Exposure time did not influence this difference (p= 0.6209). The results of the paired comparison of the groups is illustrated in Chart 1.
Therefore, we detected a difference in behavior pattern of groups 2 and 4 related to the response presented in inflammatory infiltrate regardless of exposure time.
The study of proliferation of intraepithelial glands showed some differences of responses of individuals with different exposure time (p= 0.0263) and different groups (p= 0.1425). Results of the comparison of each two groups are displayed in Chart 2.
Thus, it was detected a difference in behavior pattern of groups 1 and 3 and 1 and 4 related to the response of proliferation of intraepithelial glands regardless of exposure time.
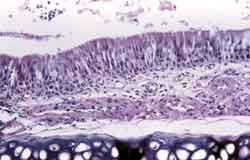
Figure 1. Nasal septum wall with pseudostratified ciliate columnar epithelium, with normal aspect. See below the submucosa of the hyaline septal cartilage. HE, 200x.
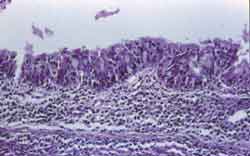
Figure 2. Pseudostratified columnar epithelium with focal anfractuous aspect, mild thickening due to gland-like proliferation. A moderate lymph plasmocitary inflammatory infiltrate is observed in the submucosa. HE, 200x.
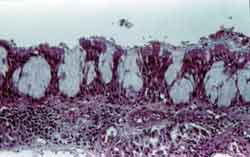
Figure 3. Intense proliferation of intraepithelial glands with thickening of epithelial lining. Submucosa with marked lymph plasmocitary inflammatory infiltrate. HE, 200x.
Chart 1. Comparison of every two groups of animals regarding inflammatory infiltrate.
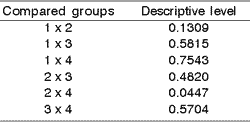
Chart 2. Comparison of every two groups of animals regarding proliferation of intraepithelial glands.
Beneficial effects of nasal irrigation in patients with rhinosinusitis or in the postoperative care of nasosinusal surgeries are well known4,7,21,22 and seem to justify the frequent indication of intranasal irrigation. The procedure involved the use of different hydroelectrolytic solutions at different concentrations and formulations, and saline solution at 0.9% is most commonly recommended. In this study, the evaluation of the effects of the application of such solution showed some local deleterious effects. Group 1 was treated with saline solution at 0.9% (solution # 1), and was the group that showed an increased level of intraepithelial gland proliferation, showing statically significant difference against group 3 and control group regardless of exposure time. Similar effect of mucosa cells have been reported in rats subject to topical treatment with different substances 17,18,20.
In the attempt to find a physiological explanation for this result, we tried to analyze the composition of the ions present in the mucus related to the different solutions investigated.
The observation of nasal mucus composition should consider saline solution at 0.9% as hypotonic, since its osmolarity is 300mOsm/l and osmolarity of normal mucus of the trachea of human beings is approximately 390mOsm/l23. In ferret's trachea values found were approximately 340mOsmol/l24 and in dog's trachea such the value was 339mOsmol/l25.
The results of this study suggest that the difference in tonicity between saline solution at 0.9% and nasal mucus could have a deleterious effect on the cells, stimulating intraepithelial gland proliferation. Some recent studies showed decrease in cilia beating of fragments of mucosa preserved with cryotherapy in the sphenoidal sinuses of human beings and the trachea of poultry embryos 27 that were exposed to this solution. These results may explain clinical results that were not very favorable regarding mucociliary clearance found by several authors that used saline solution at 0.9% for intranasal irrigation in patients without history of significant nasosinusal disease11,14, patients with acute bacterial rhinosinusitis and in patients after septoplasty 12.
Nonetheless, some authors did not find any epithelial abnormalities similar to those found in this study in the evaluation of control groups treated with saline solution at 0.9%18,20, and this could be explained by the number of sampled patients20.
On the other hand, animals that received nasal irrigation with solution 3 did not present deleterious adverse effects in nasal mucosa, since in relation to the control group it did not present significant difference in intensity of the infiltrate of inflammatory cells nor the proliferation of intraepithelial glands. This was a hypertonic solution in comparison to the components of the mucus and its osmolarity was 474mOsm/l. The results of some recent studies also suggested that hypertonicity might be a desirable characteristic for the solutions to be used in intranasal irrigation.
Nevertheless it was found that although hypertonicity could be a factor that played a positive role, it should not be marked, since some published studies showed that solutions with high hypertonicity may cause undesirable local effects such as reversible ciliastasis in the mucosa of the sphenoid sinus after 5 minutes exposed to saline solution at 7%, and irreversible ciliastasis after exposure to a solution at 14.4% 6. In addition to cilia beating, it was also observed in vitro disjunction of epithelial cells in a fragment subjected to hypertonic saline solutions at 3% and 7%28. Based on these data the solution with hypertonic characteristics was used, but with low saline concentration level.
Group 2 that received glucose-containing solutions did not present any differences compared to the control group regarding proliferation of intraepithelial glands, suggesting that this component did not influence such change in the mucosa. Solution 2 was also hyperosmolar against components of mucus (492mOsm/l), but more than half of this value was due to glucose that in higher concentrations seemed to cause marked local irritation, evidenced by increased quantity of inflammatory cells present in nasal mucosa of this group, a result that was statistically different from the other groups, including the control group, regardless of exposure time. Some laboratory rats could present inflammatory infiltrate of nasal mucosa even if they were disease-free and without being subject to an experiment 17, but depending on irritating factors, these cells tend to increase in number 18,20.
Potassium is an ion that is present in intra and extra-cellular fluids, as well as in nose mucus. Potassium-containing solutions such as Ringer with Lactate (0.030g of Potassium Chloride in 100ml of solution) and Locke-Ringer solution (with 0.042g in 100ml) were used in studies involving nasal mucosa with favorable results12,26,29. The results of this study suggested that the presence of this ion was not a determinant factor in any of the changes found in the mucosa.
Thus, it could be assumed that among the investigated solutions, the hypertonic solution, or solution # 3, is the one that could bring more benefits regarding the aspects related to nasal irrigation, and could be used without causing significant level of nasal inflammation nor proliferation of intraepithelial glands providing at the same time all benefits resulting from nasal irrigation. These benefits include faster improvement of clinical symptoms of patients, and endoscopic aspects, since desired mechanical effect of nasal lavage would be achieved without any local side effects.
Nonetheless, clinical trials in human beings are required to confirm the benefits of nasal irrigation with this solution in which we could observe the symptomatic effects of the procedure.
CONCLUSIONSThe analysis of the results of this study allowed us to conclude that:
1. Saline solution at 0.9%, or solution # 1, was improper to be used in nasal irrigation.
2. Hypertonic solution containing higher concentration of glucose, or solution # 2 was improper to be used in intra-nasal irrigation.
3. Hypertonic saline solution, or solution # 3, was the one that best favored normal functioning of mucosa, proving itself to be a proper solution to be used in intra-nasal irrigation.
REFERENCES 1. Kaliner M. Allergy Care in the Next Millennium: Guidelines fo the Specialty. J Allergy Clin Immunol 1997;99:729-34.
2. Kaliner M. Medical Management of Sinusitis. Am J Med Sci 1998;316(1):21-8.
3. Brook I, Gooch III WM, Jenkins SG, Pichichero ME, Reiner SA, Sher L, Yamauchi T. Medical Managment of Acute Bacterial Sinusitis. Ann Otol Rhinol Laryngol 2000;109 Suppl 182(5 Pt 3):2-20.
4. Tomooka, LT; Murphy, C; Davidson, TM. Clinical Study and Literature Review of Nasal Irrigation. Laryngoscope 2000;110:1189-93.
5. Burns JL. Nasal Lavage. J Otolaryngol 1992;21(2):83.
6. Sociedade Brasileira de Otorrinolaringologia. I Consenso Brasileiro Sobre Rinossinusites. Rev Bras Otorrinolaringol 1999;65(3)Supl 9:30 p.
7. Bachmann G, Hommel G, Michel O. Effect of Irrigation of the Nose with Isotonic Salt Solution on Adult Patients with Chronic Paranasal Sinus Disease. Eur Arch Otorhinolaryngol 2000;257(10):537-41.
8. Sociedade Brasileira de Otorrinolaringologia. Consenso Sobre Rinites. Rev Bras Otorrinolaringol 2000;66(3)Supl 10.
9. Nuutinen, J, Holopainen, E, Haahtela, T, Ruoppi, P, Silvasti, M. Balanced physiological saline in the treatment of chronic rhinitis. Rhinology 1986;24:265-9.
10. Kern EB. Nasal Obstruction. In: Meyerhoff WL, Rice DH. Otolaryngology - Head and Neck Surgery. Philadelphia: W.B. Saunders, 1992.p.477-506.
11. Talbot AR, Herr TM, Parsons DS. Mucociliary Clearance and Buffered Hypertonic Saline Solution. Laryngoscope 1997;107:500-3.
12. Únal M, Görür K, Özcan C. Ringer-lactate Solution Versus Isotonic Saline Solution on Mucociliary Function After Nasal Septal Surgery. J Laryngol Otol 2001;115(10);796-7.
13. Schwartz RH. The Nasal Saline Flush Procedure. Pediatr Infect Dis J 1997;16(7):725.
14. Homer JJ, Dowley AC, Condon L, El-Jassar P, Sood S. The Effect of Hypertonicity on Nasal Mucociliary Clearance. Clin Otolaryngol 2000;25, 558-60.
15. Taccariello M, Parikh Abhi, Darby Y, Scadding G. Nasal Douching as a Valuable Adjunt in the Management of Chronic Rhinosinusitis. Rhinology 1999;37:29-32.
16. Inanh S, Öztürk O, Korkmaz M, Tutkun A, Batman Ç. The Effects of Topical Agents of Fluticasone Propionate, Oxymetazoline, and 3% and 0,9% Sodium Chloride Solutions on Mucociliary Clearance in the Therapy of Acute Bacterial Rhinosinusitis In Vivo. Laryngoscope 2002;112:320-5.
17. Kelemen G, Sargent F. Non Experimental Pathologic Nasal Findings in Laboratory Rats. Archs Otolar 1946;44(1):24-42.
18. Shimizu T, Takahashi Y, Kawaguchi S, Sakakura Y. Hypertrophic and Metaplastic Changes of Goblet Cells in Rat Nasal Epithelium Induced by Endotoxin. Am J Respir Crit Care Med 1996; 53:1412-8.
19. Harkema JR, Morgan KT. Proliferative and Metaplastic Lesions in Nonolfactory Nasal Epithelia Induced by Inhaled Chemicals. In: Jones TC, Dungworth DL, Mohr U. Respiratory System. 2nd ed. New York: Springer; 1996.p.3-26.
20. Cho JL, Kwun YS, Jang HS, Kang JM, Won YS, Yoon HR. Long Term Use of Preservatives on Rat Nasal Respiratory Mucosa: Effects of Benzalkonium Chloride and Potassium Sorbate. Laryngoscope 2000;110:312-7.
21. Shoseyov D, Bibi H, Shai P, Shoseyov N, Shazberg G, Hurvitz H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. J Allergy Clin Immunol 1998;101(5):602-5.
22. Wiikmann C, Chung D, Lorenzetti F, Lessa M, Voegels R, Butugan O. Comparação entre a Solução Salina Fisiológica e a Hipertônica Tamponada após Cirurgia Endoscópica Nasossinusal. Arquivos de Otorrinolaringologia (Archives of Otorhinolaryngology) 2002;6(2):98-102.
23. Matthews LW, Spector S, Lemm J, Potter JL. Studies on Pulmonary Secretions. Am Rev Respir Dis 1963;88:199-204.
24. Robinson NP, Kyle H, Webber SE, Widdicombe JG. Eletrolyte and Other Chemical Concentrations in Tracheal Airway Surface Liquid and Mucus. J Appl Physiol 1989; 66(5): 2129-35.
25. Man SFP, Adams III GK, Proctor DF. Effects of Temperature, Relative Humidity, and Mode of Breathing on Canine Airway Secretions. J Appl Physiol 1979;46(2):205-10.
26. Boek WM, Keles N, Graamans K, Huizing EH. Physiologic and Hypertonic Saline Solutions Impair Ciliary Activity in Vitro. Laryngoscope 1999;109:396-9.
27. Boek WM, Romeijn SG, Graamans K, Verhoef JC, Merkus FWHM, Huizing EH. Validation of Animal Experiments on Ciliary Function In Vitro. II. The Influence of Absorption Enhancers, Preservatives and Physiologic Saline. Acta Otolaryngol (Stockh) 1999; 119(1):98-101.
28. Min YG, Lee KS, Yun JB, Rhee CS, Rhyoo C, Koh YY, Yi WJ Park KS. Hypertonic Saline Decreases Ciliary Movement in Human Nasal Epithelium in Vitro. Otolaryngology - Head and Neck Surgery 2001;124(3):313-6.
29. Merkus P, Romeijn SG, Verhoef JC, Merkus FW, Schouwenburg PF. Classification of Cilio-Inhibiting Effects of Nasal Drugs. Laryngoscope 2001;111(4 Pt 1):595-602.
1 Pós-Graduanda do Departamento de Otorrinolaringologia da Universidade Federal de São Paulo - Escola Paulista de Medicina.
2 Professor Doutor Afiliado da Disciplina de Otorrinolaringologia Pediátrica do Departamento de Otorrinolaringologia da
Universidade Federal de São Paulo - Escola Paulista de Medicina.
3 Doutor em Medicina pelo Departamento de Otorrinolaringologia da Universidade Federal de São Paulo - Escola Paulista de Medicina.
4 Professor Doutor Adjunto da Disciplina de Patologia Cirúrgica do Departamento de Patologia da Universidade Federal de São Paulo - Escola Paulista de Medicina.
Trabalho vinculado à Universidade Federal de São Paulo - Escola Paulista de Medicina.
Endereço para Correspondência: Erika Yokoingawa Camargo Viertler - Rua Pintassilgo, 185 apto 174 bloco 2 Moema 04514-030 São Paulo SP
Article submitted on March 12, 2003. Article accepted on June 05, 2003.


