INTRODUCTIONExtra-thyroid extension is considered a factor of poor prognosis in various systems proposed for well-differentiated thyroid carcinoma: European Organization for Research on Treatment of Cancer (1979)1; AMES (1979)2; DAMES (1984)3; AGES (1986)4; Memorial Sloan-Kettering Cancer Center (1992)5; and MACIS (1993)6. Laryngo-tracheal invasion is a significant prognostic factor, regardless of survival7. Direct invasion of adjacent tissues is present in 21% of the patients with thyroid cancer and most of them consists of well-differentiated lesions. The factors that adversely affect survival include age, male gender, and extensive involvement of neck surrounding structures8. Papillary carcinoma is the most common thyroid cancer and has the best prognosis of them all, but unfortunately they are prone to local invasion9. The invasion may be by direct extension or by extra-capsular invasion of the involved paratracheal lymph node10.
Radical removal of a thyroid carcinoma that invades the trachea and other structures is a serious problem. Complete excision can take to high surgical morbidity in the upper airways invaded site. The purpose of the present study is to retrospectively analyze the surgical results in a population of patients with locally invasive papillary thyroid carcinoma.
MATERIAL AND METHODWe have retrospectively analyzed 509 surgically treated patients at the Department of Otorhinolaryngology and Head and Neck Surgery at the Metropolitan University of Santos by thyroidectomy (partial or total), from 1994 to 2000. There were 71 cases of papillary carcinoma in the period, amounting to 13.9% of the total number of operated cases. We found among the papillary thyroid cancer cases, 13 patients with extrathyroidal extension - 18.3%.
Five patients were male and eight were female subjects. The median age was 57 years, ranging from 13 to 87 years. No patient had been submitted to previous radiotherapy treatment. Two patients had been previously operated - 3 and 12 years before the admission to our Department. Both had local recurrence. All patients complained of neck mass, slow and progressive growth and no phlogistic signs, whereas two of them had respiratory discomfort, with stridor, and one of them had intercostal draught.
In addition to the complete clinical examination, videolaryngoscopy and Computed tomography scan (CT) were performed in all patients (Figure 1). Simple chest x-ray was performed all in order to have preoperative assessment and lung staging.
In all cases, we conducted total thyroidectomy, with radical neck dissection modified in 5 cases (with preservation of spinal nerve in 5 and contralateral internal jugular vein in 3) and level VI dissection in 6 patients. One patient was submitted to sternotomy for resection of mediastinal invasion of tracheal rings. End-to-end anastomosis of the trachea was made in 5 patients (Figure 2), whereas anastomosis of cricoid cartilage and trachea was conducted in one case. Total laryngectomy was performed in one patient.
RESULTSThe most commonly invaded structures were: strap muscles in 10 cases; trachea in 9 cases; recurrent laryngeal nerve in 5 cases; larynx in 4 cases; esophagus in 1 case. The skin was free in all cases.
Surgical treatment in two patients with respiratory failure was sped up. Simple chest x-rays and clinical examination did not show signs of distance metastases in our sample.
One patient died on the 10th postoperative day owing to complications of congestive heart failure. Another patient died owing to clinical causes on the 14th postoperative day. One patient refused total laryngectomy and is alive and with the disease. This patient was submitted to incomplete tumor resection, with clear persistence of macroscopic tumor and adjuvant treatment with therapeutic dose of 131I. Eight patients progressed without signs of recurrence in a period that varied from 10 to 49 postoperative months, with mean follow-up of 26 months. One presented local recurrence 17 months after surgery and was submitted to salvage surgery. There is no further evidence of the disease after 10 months. That was one of the two patients who had been previously submitted to surgery.
Therapeutic dose of 131I was administered to all 12 patients who survived the initial postoperative period. The doses ranged from 100 to 150 mCi. After that, all patients started to receive hormonal replacement with thyroxin at suppressive doses to keep the thyrotrophin at undetectable levels, if possible. Our follow-up routine was thyroglobulin dosage every 4 months and any increase in levels required whole body investigation with 131I. The last 5 patients were submitted to adjuvant radiotherapy with median dose of 60Gy, including the surgical bed and neck and upper mediastinal lymphatic drainage fields.
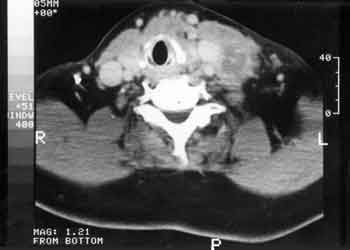
Figure 1. Coronal CT scans showing an extensive thyroid tumor invading the upper respiratory tract.

Figure 2. A thyroid tumor was resected with tracheal rings and neck dissection.
Despite the fact that there were no symptoms at the beginning of the clinical picture, with progression of the tumor, dysphonia, cough, hemoptysis and dyspnea may manifest11. Upper airway obstruction results from a combination of paralysis in one or both vocal folds with constriction of extrinsic or intraluminal mass12, 13. When the neck mass is not obvious, the aerodigestive complaints of the patient are frequently mistaken by other diseases, however, the thyroid tumor should be considered as a possible cause14. Endoscopic examination in the office provides information to assess the thyroid carcinoma. Vocal fold paralysis can be immediately detected by laryngoscopy and intraluminal invasion can be visualized, similarly to the occasional site of hemoptysis that can be determined15. Bronchoscopy should be routine part of preoperative investigation16. Simple x-ray can show compression, upper trachea stenosis and intraluminal mass17. CT scan demonstrates the affection of the involved structures and not the thyroid lesion18. Pre-thyroid or strap muscles are the most commonly invaded structures owing to their great proximity with the anterior surface of the thyroid, even though it is not a difficulty when completely removing the tumor19.
In a series of 801 patients with well-differentiated cancer of the thyroid20 32 patients had invasive tumor and represented 4% of the sample. In patients that have bulky tumors, local recurrence and death by asphyxia or uncontrollable hemorrhage seem to be a sequence of inappropriate initial treatment or absence of treatment. In a group of 70 patients who died, 61 presented residual tumor of glandular area and/or neck lymph nodes21. Asphyxia was the immediate cause of death in 26 (38.5%) of the cases22, 23. In another series, local and regional recurrence was not as frequent and most of the patients died by distance metastases or other causes rather than cancer24. Among the patients below 45 years of age, the presence of extrathyroidal invasion does not have a negative impact over survival if the primary tumor is completely resected25.
Well-differentiated carcinomas present a relatively slow growth pattern, with less aggressive behavior and favorable prognosis26. Owing to the slow growth and indolent nature, many surgeons resist performing more extensive local resections, especially in the presence of local invasion of neighboring structures27. Thus, most patients that have an apparent tracheal involvement can undergo more conservative resections with complete removal of the macroscopic disease, preservation of the larynx and trachea, with good local control of the disease11. Little differentiation at reassessment of the histopathologic findings after surgery represents worse prognosis28.
Conversely, since the most important prognostic factor is local invasion, the performance of tracheal resection has a close correlation with the progression of these patients29, 30 and surgical removal of the larynx because of local tumor invasion enables satisfactory results in the long run31, 32, 33. Tumor curettage from the trachea and therapeutic dose of iodine (131I) or external radiotherapy for control of residual tumor frequently result in late recurrence manifestation34, 35.
One segment of the airways can be removed if invaded by the tumor, including the larynx36. Many times it is necessary to remove the lower laryngeal nerve owing to tumor invasion37.
Partial excision of the anterior wall of the trachea can be easily reconstructed with a muscle-perichondral combined flap, providing wide airways and good vocal quality31. More than 25% of the cricoid cartilage can be resected without reconstruction and primary thyro-tracheal anastomosis is useful in reconstructing the patients that require removal of the whole anterior arch of the cricoid 38. Bilateral recurrent nerve paralysis is more commonly associated with laryngo-tracheal anastomosis than tracheal-tracheal anastomosis owing to the extension of the disease39.
There is a risk of recurrence in patients with subtotal excision of tumor, but the risk can be eliminated by adding external radiotherapy at the appropriate dose (4,000cGy from 3 to 3.5 weeks)40. More complete regressions can be obtained with greater doses of radiation. Such treatment is not justified as palliative nature41. We started to use adjuvant radiotherapy as a routine after 1997, in cases not previously irradiated by guidance of the referring service. Laser therapy is recommended to control minor intraluminal recurrences after conservative surgery16.
When there is a true invasion of the aerodigestive tract or the cartilage was destroyed, complete resection is the treatment of choice. However, there is still controversy concerning the resection and significant morbidity. Airways, voice and swallowing are functions that should be preserved by conservative techniques. These structures should be sacrificed only if the conservative technique is going to leave behind a residual lesion that is clearly observable. There are three definite criteria to perform curettage excision7: the tumor is firmly adhered to the wall of the superior aerodigestive tract; the removal of the tumor includes part of the wall of the involved structure without residual macroscopic disease, and a clear microscopic margin is not identified and should be presumably positive.
When the tumor is adjacent or over the tracheal or laryngeal surface, there is no need to resect the upper aerodigestive tract. Conservative resection is acceptable to preserve laryngeal functions if the residual disease is microscopic, and can be controlled by 131I or external radiotherapy. The adjuvant therapies are justified to improve prognosis. Thus, it is possible to prevent death by asphyxia or hemorrhage, have prolonged palliative relief or even reach cure. Laryngectomy can be useful in a future approach of recurrent disease. Such a surgery can be performed as initial therapy if there are no expectations of functional laryngeal preservation. Therefore, the resecting criteria of these tumors are different from those employed in squamous cell carcinoma of the upper aerodigestive tract.
Most authors agree that macroscopic residual tumor should not be left in the surgical bed. As to microscopic tumor, there are two lines - one is more conservative and the other is more aggressive. Therefore, it is important to have an active participation of the patients in the final decision of the surgical approach to be adopted.
CONCLUSIONLocally invasive thyroid papillary carcinoma should be surgically treated with the removal of the visible tumor, and we should consider with the patients the level of the surgery and quality of life depending on the structures to be resected. Adjuvant treatment is useful in the control of the disease, especially in the presence of residual lesion in the surgical bed.
REFERENCES 1. Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Silvester RJ, van Glabbeke M. A prognostic Index for Thyroid Carcinoma. A Study of The E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 1979;15:1033-41.
2. Cady B, Sedgwick CE, Meissner WA, Wool MS, Salzman FA, Werber J. Risk Factor Analysis in Differentiated Thyroid Cancer. Cancer 1979;43:810-20.
3. Cohn K, Bäckdahl M, Forsslund G, Auer G, Lundell G, Lowhagen T, Tallroth E, Willems JS, Zetterberg A, Granberg PO. Prognostic Value of Nuclear DNA Content in Papillary Thyroid Carcinoma. World J. Surg. 1984;8:474-80.
4. Hay ID, Taylor WF, McConahey WM. A prognostic score for predicting outcome in papillary thyroid carcinoma. Endocrinology 1986;119 (suppl):T-15.
5. Shah JP, Loree TR, Dharker D, Strong EW, Begg C. Prognostic Factors in Differentiated Carcinoma of the Thyroid Gland. Am J. Surg. 1992;164:658-61.
6. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993;114:1050-8.
7. Czaja JM, McCaffrey TV. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 1997;123:484-90.
8. Breaux EP, Guillamondegui OM. Treatment of locally invasive carcinoma of the thyroid: how radical? Am J Surg 1980;140:514-7.
9. Cady B. Management of tracheal obstruction from thyroid diseases. World J Surg 1982;6:696-701.
10. Czaja JM, Gluckman JL. Extended surgical procedures for invasive thyroid carcinoma in Johnson JT, Gluckman JL. Carcinoma of the thyroid, Isis Medical Media, Oxford, 1999, pp. 81-6.
11. Schinden J. Urgent extensive operation in cases of thyroid malignancy. Int Surg 1968;50:355-62.
12. Djalilian M, Beahrs OH, Devine KD, Weiland LH, DeSanto LW. Intraluminal involvement of the larynx and trachea by thyroid cancer. Am J Surg 1974;128:500-4.
13. Batsakis JG. Laryngeal involvement by thyroid disease. Ann Otol Rhinol Laryngol 1987;96:718-9.
14. Calcaterra TC, Maceri DR. Aerodigestive dysfunction secondary to thyroid tumors. Laryngoscope 1981;91:701-7.
15. McCaffrey TV, Lipton RJ. Thyroid carcinoma invading the upper aerodigestive system. Laryngoscope 1990;100:824-30.
16. Grillo HC, Suen HC, Mathisen DJ, Wain JC. Resectional management of thyroid carcinoma invading the trachea. Ann Thorac Surg 1992;54:3-10.
17. Britto E, Shah S, Parikh DM, Rao RS. Laryngotracheal invasion by well-differentiated thyroid cancer: diagnosis and management. J Surg Oncol 1990;44:25-31.
18. Fujimoto Y, Obara T, Ito Y, Kodama T, Yashiro T, Yamashita T, Nozaki M, Suzuki K. Aggressive surgical approach for locally invasive papillary carcinoma of the thyroid in patients over forty-five years of age. Surgery 1986;100:1098-107.
19. McCaffrey TV, Bergstralh EJ, Hay ID. Locally invasive papillary thyroid carcinoma: 1940-1990. Head Neck 1994;16:165-72.
20. Cody III HS, Shah JP. Locally invasive, well-differentiated thyroid cancer. Am J Surg 1981;142:480-3.
21. Tollefsen HR, DeCosse JJ, Hutter RVP. Papillary carcinoma of the thyroid. A clinical and pathological study of 70 fatal cases. Cancer 1964;17:1035-44.
22. Silliphant WM, Klinck GH, Levitin MS. Thyroid carcinoma and death: a clinicopathological study of 193 autopsies. Cancer 1964;17:513.
23. Ishihara T, Yamazaki S, Kobayashi K, Inoue H, Fukai S, Ito K, Mimura T. Resection of the trachea infiltrated by thyroid carcinoma. Ann Surg 1982;195:496-500.
24. Ballantyne AJ. Resections of the upper aerodigestive tract for locally invasive thyroid cancer. Am J Surg 1993;168:636-9.
25. Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 1995;170:467-70.
26. Tovi F, Goldstein J. Locally aggressive differentiated thyroid carcinoma. J Surg Oncol 1985;29:99-104.
27. Lipton RJ, McCaffrey TV, van Heerden JA. Surgical treatment of invasion of the upper aerodigestive tract by well-differentiated thyroid carcinoma. Am J Surg 1987;154:363-7.
28. Uemura H, Yane K, Miyahara H. Why did differentiated thyroid carcinomas with upper aerodigestive tract invasion have poor prognosis in some cases after complete dissection? Proceedings of the 1st World Congress on Head and Neck Oncology, November 1998, Madrid.
29. Nakao K, Miyata M, Izukura M, Monden Y, Maeda M, Kawashima Y. Radical operation for thyroid carcinoma invading the trachea. Arch Surg 1984;119:1046-49.
30. Tsumori T, Nakao K, Miyata M, Izukura M, Monden Y, Sakurai M, Kawashima Y, Nakahara K. Clinicopathologic study of thyroid carcinoma infiltrating the trachea. Cancer 1985;56:2843-8.
31. Friedman M, Skolnik EM, Bain HM, Becker SP, Katz AH, Mantravadi RVP. Thyroid carcinoma. Laryngoscope 1980;90:1991-2003.
32. Shvili Y, Zohar Y, Buller N, Laurian N. Conservative surgical management of invasive differentiated thyroid cancer. J Laryngol Otol 1985;99:1255-60.
33. Friedman M, Danielzadeh JA, Caldarelli DD. Treatment of patients with carcinoma of the thyroid invading the airway. Arch Otolaryngol Head Neck Surg 1994;120:1377-81.
34. Grillo HC, Zannini P. Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg 1986;42:287-98.
35. Tubiana M, Haddad E, Schlumberger M, Hill C, Rougier P, Sarrazin D. External radiotherapy in thyroid cancers. Cancer 1985;55:2062-71.
36. Chagas JFC: Carcinoma diferenciado avançado. Patologia cirúrgica da tireóide (Dedivitis RA, Guimarães AV). São Paulo: Frôntis Editorial; 1999. p. 185-92.
37. Mellière DJM, Yahia NEB, Becquemin JP, Lange F, Boulahdour H. Thyroid carcinoma with tracheal or esophageal involvement: Limited or maximal surgery? Surgery 1993;113:166-72.
38. Friedman M, Shelton VK, Skolnik EM, Berlinger FG, Arab M. Laryngotracheal invasion by thyroid carcinoma. Ann Otol Rhinol Laryngol 1982;91:363-9.
39. Ishihara T, Kobayashi K, Kikuchi K, Kato R, Kawamura M, Ito K. Surgical treatment of advanced thyroid carcinoma invading the trachea. J Thorac Cardiovasc Surg 1991;102:717-20.
40. Simpson WJ. Radiotherapy in thyroid cancer. Can Med J Assoc 1975;113:115.
41. Simpson WJ, Carruthers JS. The role of external radiation in the management of papillary and follicular thyroid cancer. Am J Surg 1978;136:457-60.
1Ph.D. in Medicine, Post-Graduation Course in Otorhinolaryngology and Head and Neck Surgery, Federal University of São Paulo/ Escola Paulista de Medicina, São Paulo. Faculty Professor, Discipline of Otorhinolaryngology and Head and Neck Surgery, Metropolitan University of Santos, Santos, Brazil.
2 Master in Medicine, Post-Graduation Course in Head and Neck Surgery Complexo Hospitalar Heliópolis, São Paulo. Assistant Professor, Discipline of Otorhinolaryngology and Head and Neck Surgery, Metropolitan University of Santos, Santos, Brasil.
Affiliation: Discipline of Otorhinolaryngology and Head and Neck Surgery, Metropolitan University of Santos 'UNIMES', Santos, and Course of Post-Graduation in Head and Neck Surgery, Complexo Hospitalar Heliópolis, São Paulo, Brazil.
Address correspondence to: Rogério A. Dedivitis - Rua Olinto Rodrigues Dantas, 343 cj. 92
Santos - SP - 11050-220 - Tel/fax (55 13) 3221-1514 / 3223-5550 - E-mail: dedivitis.hns@uol.com.br
Presented at II International Congress on Malformations and Rare Tumors of Head and Neck, held on October 18 - 21 in Avellino, Italy.
Article submitted on February 15, 2002. Article accepted on May 09, 2002


