INTRODUCTIONThe indications for external graft of the nasal dorsum are to elevate the nasal root and nasofrontal angle, especially in cases of short nasal bones, and to camouflage a defect in the middle third of the medium dorsal dorsum.
1 The preferred materials for support of the nasal dorsum should provide adequate strength, volume, and shape persistence, as well as sufficient availability and capacity to mimic the natural contour of the dorsum.
2-4 Several materials have been proposed for use in nasal reconstruction, but there is no current consensus on which is the best. Implants are divided into four categories: autologous, homologous, heterologous, and alloplastic grafts.
5 Autologous grafts, which are obtained from different locations in the body of the patient and are used for nasal reconstruction, are primarily composed of bone and cartilage. Bone can be obtained from the iliac crest, ribs, tibia, skull, and ulna. The cartilage can be obtained from the nasal septum and the auricle for reconstruction of smaller defects, and from the costal cartilage for the reconstruction of major nasal defects.
6 Homologous grafts are obtained from other individuals of the same species as the recipient and include both cartilage and bone from cadavers, as well as from patients undergoing other surgeries, and subsequently processed in order to diminish contamination and potential host rejection, for use in other individuals of the same species.
7 Heterologous grafts are obtained from individuals of a different species than the recipient, and the most frequently used graft in nasal reconstruction is bovine cartilage.
8 The different types of grafts have advantages and drawbacks. Autologous grafts have the benefit of being biocompatible and show little resorption, but may undergo deformation at a later period.
9 Alloplastic materials have the advantage of being found in different sizes and formats, and they can be molded or shaped during surgery.
8 Currently, there are several types on the market such as silicone, Mersilene
® mesh, polyethylene, Silastic
® methyl-methacrylate, Supramid
® mesh, Teflon
®, Proplast
®, hydroxyapatite, and Gore-Tex
®.
8Of the autologous tissues, cartilaginous tissue demonstrates of low antigenicity, which makes grafting feasibility to be closely associated with higher survival and adaptation of chondrocytes to the recipient site. Its nutrition occurs by direct contact with nutrients in the implant bed, as it does not require a vascular supply to maintain its functional structure. And, when compared with bone, cartilage has a lower absorption rate and can be easily shaped or molded.
Auricular cartilage is elastic, similar to hyaline cartilage, but it includes, in addition to collagen fibrils (mainly type II), an abundant network of continuous elastic fibers together with the perichondrium fibers. Elastic cartilage is specially adapted to withstand repeated flexing, grows by apposition, and is less subject to degenerative processes than the hyaline cartilage. Unlike the latter, the matrix of elastic cartilage does not calcify, except as part of the regenerative process.
10 This study intended to experimentally compare the inflammatory response over time of ear cartilage graftswith or without Gelfoam
®, when grafted on the nasal dorsum of rabbits.
LITERATURE REVIEWBelow is a summary of the chronologically retrieved articles, of the properties of Gelfoam
® contained in the leaflet regulated by the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - ANVISA),
11 as well as some particular characteristics of biomaterials.
Cottle
12 was the first author to introduce the use of compressed cartilage for reconstruction of the nasal contour.
The graft for the nasal bone can be obtained from the vomer bone, perpendicular lamina of the ethmoid bone, turbinate, and frontal process of the maxilla, according to Kosteck.
13 Peynègre et al.
14 used a mixed graft consisting of bone powder mixed with tissue glue (Tissucol
®) and reinforced with Surgicel
® to correct irregularities of the nasal dorsum. The graft was placed and positioned between the osteo-cartilaginous supporting structures and the skin with good cosmetic results.
Guerrerosantos,
15 in his studies, used auricular cartilage to augment the nasal dorsum in rhinoplasties, advocating the maintenance of the posterior surface perichondrium attached to the graft.
Jovanovic and Berghaus
16 believe that auricular grafts are almost ideal because: 1) obtaining the material is a low-risk, fast procedure that can be performed under local anesthesia; 2) the ear cartilage is stable enough to produce support and elastic enough to produce contours; 3) it is easy to mold, 4) it has little tendency to displacement, 5) there is no absorption; and 6) there is little rejection potential. Sheen
17 considers that ear cartilage is well applied to cartilaginous dorsum, tip, and stenotic vestibular areas. It is a malleable material, but not too firm. It is not absorbed with time. It should not be used when structural support is mandatory (as in the case of columellar strut or spreader grafts).
In a five-year study performed by Mitz and Maladry,
18 in which ear cartilage grafts obtained from the scapha were used for the reconstruction of the nasal dorsum or the lower lateral cartilages, the results showed moderate resorption.
Leaf
19 advocates that the autograft of subcutaneous muscular aponeurotic system (SMAS) can be used to correct nasal dorsum deformities during surgery associated with rhytidectomy, in which a part of it is usually resected.
According to Sheen,
20 one of the most feared complications is infection, with the highest rate of infection after grafting occurring when ear cartilage is used in primary rhinoplasty due to Gram-negative bacteria from the outer ear, and that it decreased from 15% to 0.5% after intraoperative care, such as soaking the graft in lincomycin or garamycin solution, careful closure of incisions, smaller incisions, and exchange of sterile material after obtaining the graft from another surgical field or prior to implant placement.
Patrocínio and Patrocinio
21 reported that the use of auricular cartilage autograft has several advantages: the second operative field is geographically closely related to the first; it is performed under local anesthesia; and it has low morbidity and minimal bending, displacement, or extrusion. The limited amount of cartilage, the additional surgical time, and different operative field, which requires separate incisions, are some of the disadvantages of this technique, in addition to a possible cosmetic deformity of the ear. It can also be used to reconstruct the alar cartilages.
Gurlek et al.
22 advocates that the use of the lower turbinate is favorable for saddle nose reconstruction, since it is easily obtainable, inexpensive, practically ready to be used, shows no resorption in the long term, and a secondary donor area is not required, in addition to increasing the passage of air, thus preventing a possible obstruction.
With these results, the authors concluded that fresh autologous cartilaginous graft was superior to homologous and autologous cartilage grafts preserved in 70% alcohol and not crushed , and that these two showed similar histological results. The grafts in crushed form showed inferior results when compared to non-crushed grafts.
The incidence of chondritis and perichondritis in the donor site of the auricular graft was extensively studied by Kaplan and Cook in 2004,
23 who evaluated 341 cases of nose and ear reconstruction. The procedures consisted of total skin grafts from the auricular concha, total skin grafts together with auricular concha cartilage, and local flaps from the nose or ear using auricular concha or anti-helix cartilage. When the perichondrium was involved, the authors used prophylactic antibiotics with coverage for Pseudomonas sp. These patients were followed-up for 12 weeks; inflammatory chondritis was observed in 5.6% of cases, and no cases of suppurative chondritis.
Çelik et al.
24 performed surgeries for correction of nasal dorsum deformities in over 60 patients using temporoparietal bone grafts associated with auricular cartilage grafts, which were interposed and joined with Spongostan
®, with good results. Gelfoam
® is broadly used in otological surgeries and now also in laryngeal surgeries; Pontes and Vieira
25 used it in a singer who had glottal insufficiency due to vocal fold atrophy. Gelfoam
® was hydrated with saline solution to form a paste, which was then applied through percutaneous and transluminal route at the office with positive results, according to the authors, allowing the patient to return to her activities and to conclude the work until its gradual absorption.
In a study performed by Costa et al.,
26 15 rabbits were assessed to compare the use of butyl-2-cyanoacrylate, gelatin-resorcinol-formaldehyde (GRF), and suture in the stabilization of cartilage grafts in rabbits, from whose ears six cartilage grafts were resected, fixed in the periosteum of the skull, and united two-by-two with suture, GRF, and cyanoacrylate. GRF was shown to be a superior method of stabilization of cartilage grafts in rabbits when compared to butyl-2-cyanoacrylate in all cases, and superior to suture in bone-cartilage fixation. Further clinical studies may also demonstrate the efficacy and safety of this adhesive material in rhinoplasties.
Espinosa et al.
27 prefer to use previously treated homologous fascia lata grafts from a tissue organ bank for nasal dorsum lifting in cases of secondary rhinoplasty.
Pochat et al.
1 reported on a series of 12 patients that underwent surgery within a period of 14 months, submitted to rhinoplasty for correction of esthetic and functional deformities. These procedures used autologous grafts of septal cartilage, auricular concha, and ribs. The most frequently used grafts were strut grafts (100%), spreader grafts (92%), alar contour grafts (58%), and lateral crural strut grafts (33%). The authors did not report any case of distress or pathological scarring. However, some complications were observed in the donor areas: one case of ear hematoma, one case of hypertrophic scar on the chest, and two cases of pustules in the septal mucosa.
The esthetic result was satisfactory for patients in 92% of cases: from the functional point of view, in 58% of cases there was an improvement in the quality of breathing, and in 42%, the function remained unchanged. With this sampling and data, these same authors concluded that the use of autogenous cartilage grafts was effective in the treatment of esthetic and functional deformities, providing adequate support to the osteo-cartilaginous skeleton, as well as to the internal and external nasal valves, with an acceptable complication rate and low morbidity of donor sites.
Tostes et al.
28 conducted a study with seven patients submitted to Gore-Tex
® implants to fill the nasal dorsum, who were followed-up for a period of two to five years. Five of these seven individuals were primary and two were secondary rhinoplasties. External rhinoplasty was performed in five cases, closed rhinoplasty in one case, and in another case an incision was made over a traumatic scar on the nasal dorsum.
The implant with Gore-Tex
® was made manually; it was cut into progressively smaller segments to form a pyramid, and stabilized with absorbable suture stitches. According to the authors, all patients attained esthetic and functional satisfaction with excellent results, when comparing the photographic documentation of pre- and postoperative results.
Souza
29 performed an experimental study aiming at comparing the use of auricular and septal cartilage regarding the aspects of absorption, granulation, and inflammation when grafted on the nasal dorsum of rabbits. In his study, the author aimed to compare the two types of cartilage in the nasal dorsum of rabbits. Twenty-eight rabbits were studied for up to six months of follow-up, with one-half for each type of cartilage. After this follow-up period, the rabbits were euthanized, and morphological and histological studies were performed; graft resorption was observed in most rabbits regardless of the donor site, with no statistical difference that justified the distinction between the types of cartilage. The replacement of grafted tissues by fibroadipose, bone, or fibrous tissue in that study was noteworthy.
Gelfoam®Gelfoam
® is a substance consisting of a sterile absorbable gelatin sponge made of porcine skin. When implanted in tissues, it is completely absorbed within four to six weeks without causing excessive scar tissue formation. When applied to hemorrhagic areas of the vaginal, rectal, nasal mucosa or skin, it liquefies completely within two to five days. It is prepared with a solution of specially treated, purified gelatin, heated until it reaches suitable porosity, and then dried, cut, packaged, sealed, and sterilized by dry heat.
30 It is indicated in surgical procedures to aid in achieving hemostasis in the following areas of surgery: neurosurgery, gynecology, orthopedics, urology, abdominal and anorectal surgery; in otolaryngology , it is used in otology, rhinology, and laryngology.
30It is contraindicated for the closure of skin incisions, as it may interfere with wound healing; moreover, it should not be employed to overcome postpartum bleeding or menorrhagia. Additionally, it is not recommended in the presence of infections and intravascular compartments due to the risk of embolism.
30BiomaterialsANVISA
11 establishes that biomaterials are synthetic or natural materials, whether solid or liquid, used in medical devices.
According to Amorim,
31 when a substance is implanted in the body there is a universal reaction, initially with formation of inflammatory tissue consisting of blood exudate cells and development of inflammatory tissue, with accumulation of biopolymers of connective tissue of the extracellular matrix. The process is completed with the formation of collagen-rich fibrous tissue, leading to anatomical and biomechanical repair of the defect. These results depend on certain characteristics of the material, such as porosity, surface texture, consistency, and physicochemical properties.
METHODS
MaterialThe present study used 30 adult New Zealand rabbits, weighing approximately 4 kg; sterile Gelfoam
®; 3.0 mononylon thread; lidocaine with adrenaline, 1:200,000; aqueous Chlorhexidine
®; a No. 15 scalpel blade; and a surgical rhinoplasty set consisting of tweezers, needle holder, scissors, aspirator, surgical compass, Africht retractor, and insulin syringe and needle.
Sample selection and sizeThe rabbits were randomly assigned into two groups by flipping a coin: the group without Gelfoam
®, consisting of 15 rabbits, and the group with Gelfoam
®, consisting of 15 rabbits. The next step was to divide them into three subgroups as follows:
Group 1: Ten rabbits followed for seven days after the procedure; five with auricular cartilage, and five with auricular cartilage using Gelfoam®.
Group 2: Ten rabbits followed for 30 days after the procedure; five with auricular cartilage, and five with auricular cartilage using Gelfoam®.
Group 3: Ten rabbits followed for 60 days after the procedure; five with auricular cartilage, and five with auricular cartilage using Gelfoam®.
Surgical procedureAfter approval by the Ethics Committee on Animal Trials, under number 030/10, the surgical procedures were performed in accordance with the ethical principles of animal experimentation established by the Brazilian Code of Experimentation on Animals (Código Brasileiro de Experimentação em Animais - COBEA).
The surgical procedures were performed with the rabbits under general anesthesia, using Zoletil
® (tiletamine associated with zolazepan) and Nilperidol
® (fentanyl associated with droperidol), and maintained on spontaneous ventilation in the prone position.
The auricular cartilage was obtained after the infiltration of an anesthetic with vasoconstrictor agent (Fig. 1); a rectangular incision was made on the skin of the pinna of approximately 3 cm in length after subcutaneous and perichondrium dissection (Fig. 2).
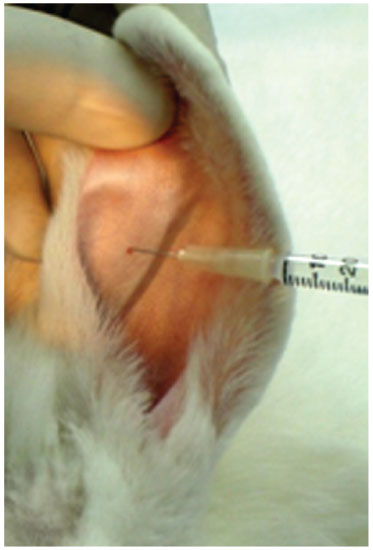
Figure 1 Ear infiltration with 2% lidocaine and norepinephrine vasoconstrictor, at a concentration of 1:200,000 (Xylocaine
®).
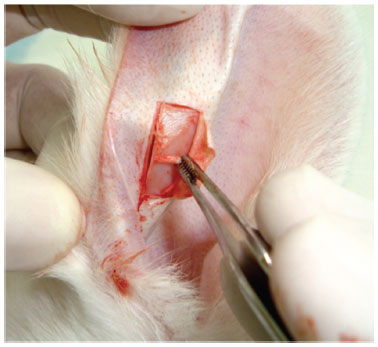
Figure 2 Horizontal incisions performed with folded skin and perichondrium removal.
To place the graft on the nasal dorsum, an infiltration was performed in the interocular region, and then a centralized incision of approximately 1 cm in horizontal extension was performed (Fig. 3). A subperiosteal tunnel, 3 cm long and 1 cm wide, was made from the interocular region to the nasal tip to serve as a pouch for graft placement.
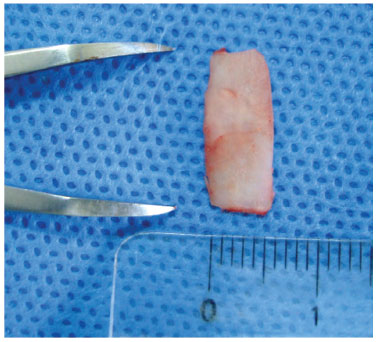
Figure 3 Preparing a 0.5-cm wide strip of cartilage, measured with a surgical compass without Gelfoam
®.
After establishing the graft placement position, a strip, 1.5 cm long by 0.5 cm wide, was drawn and cut out from the auricular cartilage, measured with a surgical compass for placement of the graft pocket on the nasal dorsum, with half with Gelfoam
® (Fig. 4) and half without Gelfoam
® (Fig. 5).
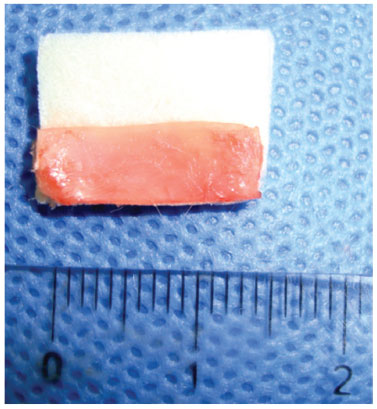
Figure 4 Preparing a 1.5-cm long strip of cartilage, measured with a surgical compass without Gelfoam
®.
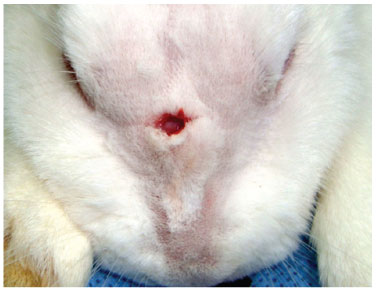
Figure 5 Centralized incision, approximately 1 cm in extension.
After the cartilage strip was inserted into the pouch (Fig. 6), a 3.0 mononylon thread stitch was applied to the eyebrow incision and at the pinna, where the material was collected, and the procedure was terminated.
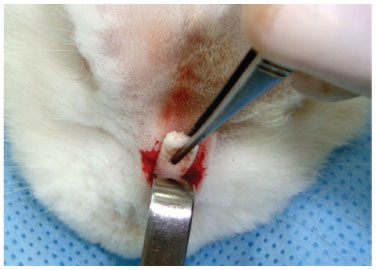
Figure 6 Subperiosteal tunnel, approximately 3 cm long and 1 cm wide, with insertion of cartilage with Gelfoam
®.
The rabbits were maintained in subgroups under the same pre-treatment daily care conditions for seven, 30, and 60 days, when they were euthanized and submitted to histopathological assessment of the surgical specimen.
For euthanization at the end of the follow-up period, the rabbits were again anesthetized and given sodium thiopental IV (40 mg/kg). After euthanization, the facial mesostructure was removed en bloc for histological study.
The rabbits were assessed by clinical parameters that could indirectly evaluate conditions of tolerability, such as general discomfort and breathing. The parameters evaluated were: food intake, weight variation, temperature, respiratory rate, and presence of nasal bleeding. All rabbits were weighed before the procedure and daily until euthanization. Food intake was monitored daily in grams. Auricular temperature was measured twice a day in degrees Celsius. Respiratory rate was recorded twice daily.
Facial mesostructures of the animals were dissected and fixed in formalin 10%, and then sent to the anatomopathological laboratory for analysis by an experienced pathologist.
Histological analysisThe facial mesostructures of animals were immersed for five days in a 5% nitric acid solution for decalcification. Once decalcified, serial cross-sectional sections of the nose and skull were made at every 5 mm. The slices were then dehydrated, clarified, and embedded in paraffin. The specimens were then cut with a microtome at an average thickness of 5µm.
The sections were stained with hematoxylin-eosin, and tissue response patterns were evaluated histologically regarding: presence of neovascularization, granulation tissue, acute inflammation (neutrophils, eosinophils and fibrin) and chronic inflammation (fibroblasts, macrophages, lymphocytes, histiocytes, and plasmocytes). The slides were analyzed by a single pathologist blinded to which group the specimen belonged to, and classified into grades using qualitative, semiquantitative criteria. Therefore, tissue response patterns were considered mild (+), moderate (+ +) and intense (+ + +) (Figs. 7 and 8).
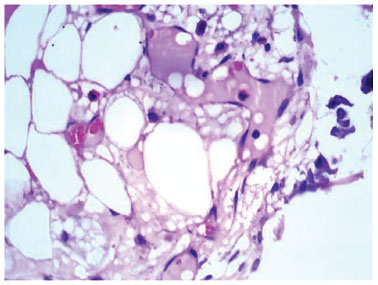
Figure 7 Optical microscopy photomicrograph showing acute inflammatory cells. Hematoxylin and eosin staining. Magnification: 100×.
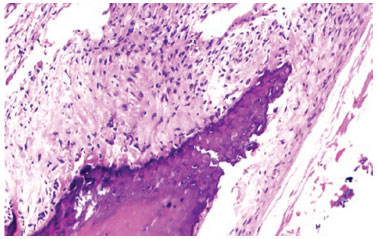
Figure 8 Optical microscopy photomicrograph showing chronic inflammatory cells. Hematoxylin and eosin staining. Magnification: 40×.
The chi-squared or Fisher's exact test was used to compare neovascularization, formation of granulation tissue, and acute and chronic inflammation between the groups. The level of significance was set at 5% (0.05).
RESULTSNone of the rabbits showed any surgical or postoperative complications.
The vast majority showed slight weight loss on the first day after the procedure, subsequently showing progressive weight gain, which was expected for the period.
Table 1 shows the mean weight of the rabbits in both groups before and after the surgical procedure.
Fig. 9 shows that there was no statistical difference regarding the presence of neovascularization between the groups with and without Gelfoam
®, when compared through the chi-squared or Fisher's exact test, with a significance level of 5% (0.05).
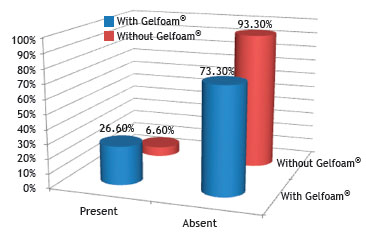
Figure 9 Percentage of rabbits that showed neovascularization, in the control (without Gelfoam
®) and experimental (with Gelfoam
®) groups.
Fig. 10 shows no statistical difference regarding the presence of granulation tissue in the group with and the group without Gelfoam
®, when compared through the chi-squared or Fisher's exact test, with a significance level of 5% (0.05).
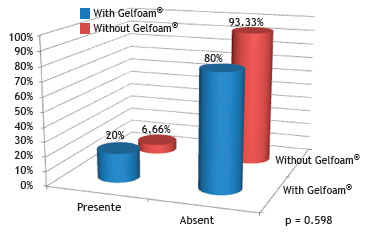
Figure 10 Percentage of rabbits that had granulation tissue in the control (without Gelfoam
®) and experimental (with Gelfoam
®) groups.
Fig. 11 shows no statistical difference regarding the presence of an acute inflammatory process in the groups without and with Gelfoam
® when compared by the chi-squared or Fisher's exact test, with a significance level of 5% (0.05).
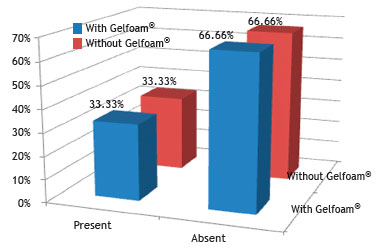
Figure 11 Percentage of rabbits that had acute inflammatory process in the control (without Gelfoam
®) and experimental (with Gelfoam
®) groups.
Fig. 12 shows no statistical difference regarding the presence of chronic inflammatory process in the groups without and with Gelfoam
®, when compared by the chi-squared or Fisher's exact test, with a significance level of 5% (0.05).
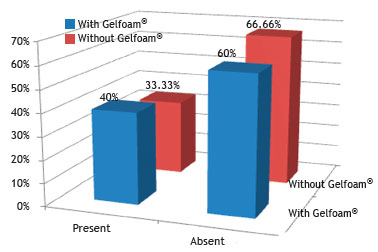
Figure 12 Percentage of rabbits that had acute inflammatory process in the control (without Gelfoam
®) and experimental (with Gelfoam
®) groups.
Among the several options graft for nasal reconstruction, in this study auricular cartilage was chosen, which especially considers situations where septal cartilage is no longer available, such as cases of secondary rhinoplasty and large septal perforations due to several reasons (iatrogenic, traumas, consequences of septal hematoma, granulomatous diseases, cocaine users, among others).
The large number of surgical techniques for nasal dorsum reconstruction described in the literature and the numerous materials that can be used suggest that the best technique still remains controversial.
The reason for choosing Gelfoam
® was that otolaryngologists already have practice and experience with its use, especially in otological surgeries and in some laryngeal cases. The time determined in this study was due to the fact that the biomaterial leaflet stated that, when in contact with blood, it would dissolve in approximately two to five days, but when in contact with tissue, it could remain intact for four to six weeks. As this experiment did not result in abundant bleeding, it was decided to test it on scar tissue for seven, 30, and 60 days. Moreover, it is known that there is a degree of absorption of the grafted material, and the aim of the study was to place something with the expectation of more scar tissue formation in the recipient area.
Currently, the grafts and implants most often used include: autogenous cartilage and bone, which, although preferred, are not always available, due to previous surgery in the affected region, or technical difficulty in obtaining them. Moreover, discomfort and possible complications at the donor site are important factor. Synthetic or alloplastic materials are also not devoid of problems, as infection and graft extrusion may occur. To test a possible new compound for nasal grafting, an experimental study using New Zealand rabbits was designed.
To develop the model of nasal dorsum reconstruction in rabbits with and without Gelfoam
®, New Zealand rabbits were chosen, as they have an abundant auricular cartilage donor area. Another advantage is the size of the animal, allowing for its handling, transportation, storage, and maintenance in small cages.
Due to its docile character, animal handling during the preoperative, operative, and postoperative steps was simple. In order to reduce the possibility of infection, all animals were submitted to strict antiseptic procedures.
The researcher was careful to remove the perichondrium from all cartilage grafts, so that in the future it can be compared, with the presence of perichondrium, respecting the length of 1.5 cm by width of 0.5 cm, the same measures that were used for those with Gelfoam
®.
Some authors have reported the use of several different grafts for correction of saddle nose from distant regions such as fascia lata,
27 temporoparietal bone,
24 inferior turbinate,
23 subcutaneous muscular aponeurotic system (SMAS),
19 without taking into consideration the numerous alloplastic grafts, as described by Sheen,
17 Costa et al.,
26 and Tostes et al.
28 In spite of the advantages associated with the use of auricular cartilage as the donor area, some surgeons are reluctant to use it due to concerns about potential infectious complications during cartilage manipulation. Tissue ischemia could lead to the development of suppurative chondritis. The necrosis that would follow could produce esthetic, psychological, and forensic implications.
23 Fortunately, in the present study, no such problems were observed, as there was a great concern regarding aseptic and antiseptic procedures, as well as the use of prophylactic antibiotics, as observed in the work by Jovanovic and Berghaus
16 and Souza.
29 The frequency of complications in the auricular donor area, associated with the manipulation of the skin and cartilage for reconstructive procedures, is minimal when the technique is sterile, delicate, and appropriate prophylactic antibiotics are used, according to Patrocínio and Patrocinio
21 and Kaplan and Cook.
23 Regarding the presence of neovascularization, only one rabbit from the group without Gelfoam
® showed it, whereas 20% of the group with Gelfoam
® demonstrated the presence of vascular network. Only rabbits from groups I and II showed neovascularization, albeit only mild and moderate.
The appearance of giant cells characterizes the granulation reaction. In the group with Gelfoam
®, slightly more than 25% showed granulation tissue. None of the animals in group III had granulation tissue; however, all levels of intensity were observed in group I, and only mild intensity was observed in two rabbits from group II.
The finding that only the seven and 30-day groups showed neovascularization and granulation tissue is because this part of the inflammatory response evolution often appears and disappears simultaneously, and gradually decreases over time until it completely disappears; for this reason, the histopathological analysis of the group after 60 days no longer showed the presence of neovascularization and granulation tissue.
The acute inflammatory process was the same in both groups, with five rabbits from each; as for the chronic inflammation process, there were five rabbits from the control group and six rabbits from the experimental group. There was acute inflammatory reaction in all three groups, but only one rabbit from group I showed an intense response. The same occurred for chronic inflammatory reaction; however, none of the animals showed intense response, only mild and moderate responses.
This research was not the first to use some type of biomaterial in combination with an autologous graft for correction of nasal dorsum. In 1990, Peynègre et al.
14 mixed bone with Tissucol
® and reinforced with Surgicel
® to correct nasal dorsum irregularities. Çelik et al.
24 used pieces of temporoparietal bone associated with interposed auricular cartilage and joined with Spongostam
®, also for the correction of saddle nose.
In the present study, the graft without the perichondrium was chosen, similar to Souza,
29 but unlike the studies by Guerrerosantos,
15 who used the perichondrium attached to the graft, and by Gurlek et al.,
23 who left the bone of the inferior turbinate with an intact periosteum for corrections of saddle nose.
The researcher chose to use an intact graft piece, which was also observed in other studies, such as those by Sheen
17 and Souza;
29 however, some authors, such as Cottle,
15 prefer the use of crushed grafts.
Doner et al.,
10 determined that the best material for graft fixation would be cyanoacrylate instead of sutures, whereas Costa et al.
26 compared graft stabilization with butyl-2-cyanoacrylate, GRF and sutures. However, in the present study, it was decided not to fix the grafts, similarly to other studies by Peynégre et al,
14 Gurlek et al.,
22 and Souza.
29 As final considerations, it is known that grafting techniques have increased the possibilities for surgeons and the expectations regarding success in rhinoplasty. When considering the indications concerning the type of graft and its place of use, as well as the individual characteristics of the patient, these constitute an excellent resource for filling or modification of the nasal framework. However, common sense indicates that the surgeon should intervene as little as necessary to attain a good result for the longest period possible.
CONCLUSIONSThere was no statistically significant difference between the groups with and without Gelfoam
® regarding neovascularization and granulation tissue formation, although groups I and II that had Gelfoam
® showed a higher tendency for these changes.
Regarding acute and chronic inflammation, there was no statistically significant difference between the two groups.
No advantages were observed regarding the use of Gelfoam
® to cover the cartilage in this study.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
REFERENCES1. Pochat VD, Alonso N, Meneses JVL. Avaliação funcional e estética da rinoplastia com enxertos cartilaginosos. Rev Bras Cir Plást. 2010;25:260-70.
2. Jackson IT, Smith J, Mixter RC. Nasal bone grafting using split skull grafts. Ann Plast Surg. 1983;11:533-40.
3. Frodel JL, Lawrence JM, Quatela VC, Weinstein GS. Calvarial bone graft harvest: techniques, considerations and morbidity. Arch Otolaryngol Head Neck Surg. 1993;119:17-23.
4. Cheney ML, Glicklich RE. The use of calvarial bone in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1995;643-8
5. Vuyk HD, Adamson PA. Biomaterials in rhinoplasty. Clin Otolaryngol. 1998;23:209-17.
6. Daniel RK. Rhinoplasty and rib grafts: evolving a flexible operative technique. Plast Reconst Surg. 1994;94:597-609
7. Ersek RA, Dalerm AG. Processed irradied bovine cartilage for nasal reconstruction. Ann Plast Surg.1988;20:540-6.
8. Mitre IE. Emprego de enxertos autólogos e homólogos em rinoplastias reconstrutivas e estéticas. Tese (Doutorado). São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 1997.
9. Staindl O. Therapy of saddle nose. Laryngol Rhinol Otol. 1983;62:348-55.
10. Doner F, Sari I, Ozturk AR, Karasen M, Bitirem M, Sutbeyaz Y. The auricular cartilage graft fixation with butyl-2cyanocrylate. Turk J Med Sci. 1998;28:285-90.
11. Agência Nacional de Vigilância Sanitária. Título do documento. Brasília, DF, 2005
12. Cottle MH. Nasal surgery in children. Ear Nose Throat Monthly. 1951;30:32-8.
13. Kosteck JL. Correction of saddle nose deformity with a turn-over hump segment procedure (case report). Plast Reconstr Surg.1966;38:372-5.
14. Peynègre R, Bossard B, Gilain L, Delacour I, Laccourreye O, Coste A, et al. Le griffon de poudre dos amalgame et arme dans La correction de l'arête nasale. Ann Oto-laryngol.1990;107:71-5.
15. Guerrerosantos J. Nose and paranasal argumentatiom: autogenous, fascia, and cartilage. Clin Plast Surg. 1991;18:65-86.
16. Jovanovic S, Berghaus A. Autogenous auricular concha cartilage transplant in corrective rhinoplasty. Practical hints and critical remarks. Rhinology. 1991;29:273-9.
17. Sheen JH. Tip graft: a 20-year retrospective. Plast Reconstr Surg. 1993;91:48.
18. Mitz V, Maladry D. Intérêt du prélèvement du scapha au cours dês rhinoplasties secondaires. Ann Chir Plast Esthét. 1996;41:68-74.
19. Leaf N. SMAS autografts for the nasal dorsum. Plast Recontr Surg. 1996;97:1249-52.
20. Sheen JH, Sheen AP. Aesthetic Rhinoplasty, 2nd ed. St Louis: Quality Medical Publishing;1998 (reprint of 1987 ed.).
21. Patrocínio LG, Patrocinio JA. Atualização em enxertos na rinoplastia. Braz J Otorhinolaryngol. 2001;67:394-402.
22. Gurlek A, Askar I, Bilen BT, Aydogan H, Fariz A, Alaybeyoglu N. The use of lower turbinate bone graft in the treatment of saddle nose deformities. Aesthetic Plast Surg. 2002;26:407-12.
23. Kaplan AL, Cook JL. The incidence of chondritis e perichondritis associated with surgical manipulation of auricular cartilage. Dermatol Surg. 2004;30:58-62.
24. Çelik M, Haliloglu T, Bayçin N. Bone chips and diced cartilage: an anatomically adopted graft for the nasal dorsum. Aesthetic Plast Surg. 2004;28:8-12.
25. Pontes PAL, Vieira VP. Aplicações de Gelfoam
® como tratamento de emergência na insuficiência glótica em cantora. Braz J Otorhinolaryngol. 2004;70:410-4.
26. Costa HJZR, Pereira CSB, Costa MP, Fabri FSSS, Lancelotti CLP, Dolci JEL. Estudo experimental comparativo entre o butil-2-cianoacrilato, a mistura gelatina-resorcina-formaldeído e sutura na estabilização de enxertos de cartilagem em coelhos. Braz J Otorhinolaryngol. 2006;72:61-70.
27. Espinosa JA, Jaramilo R. Fascia Homologa de banco en rinoplastia: reporte de casos. Acta Otorr Cir Cabeza y Cuello. 2007;35:88-92.
28. Tostes ROG, Ferreira FPM, Junior JCCGA, Lima JCSA, Almeida PN, Meira AAM, et al. Uso do Gore-tex
® para preenchimento do dorso nasal em rinoplastias. Rev Bras Cir Plast. 2011;26:461-5.
29. Souza LO. Estudo experimental e comparativo da utilização da cartilagem septal e auricular no dorso nasal de coelhos. Tese (Mestrado) São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2012.
30. Bula do Gelfoam
® Pharmacia & Upjohn Co. EUA.
31. Amorim WL. Estudo experimental da resposta tecidual a presença de celulose produzida por acetobacter xylinum. Tese (Doutorado). São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo; 2007.
1. Graz University, Austria
2. Faculdade de Ciências Médicas, Santa Casa de São Paulo, São Paulo, SP, Brazil
Corresponding author.
B.S.R. Silva
E-mail:
brenosimoes21@yahoo.com.brReceived 9 January 2013.
Accepted 10 November 2013.
* Study conducted at Hospital Santa Casa de São Paulo, São Paulo, SP, Brazil.


