AuthorsAssociação Brasileira de Otorrinolaringologia e Cirurgia Cérvico Facial (Brazilian Association of Otolaryngology and Neck and Facial Surgery)
Academia Brasileira de Neurologia (Brazilian Academy of Neurology)
Sociedade Brasileira de Cardiologia (Brazilian Society of Cardiology)
Sociedade Brasileira de Pediatria (Brazilian Society of Pediatrics)
Sociedade Brasileira de Pneumologia e Tisiologia (Brazilian Society of Pneumology and Tisiology)
ParticipantsZancanella E, Haddad FM, Oliveira LAMP, Nakasato A, Duarte BB, Soares CFP, Cahali MB, Eckeli A, Caramelli B, Drager L, Ramos BD, Nóbrega M, Fagondes SC, Andrada NC
Final developmentJune 11, 2012.
Description of the evidence collection methodAn active search was conducted in the PubMed/MEDLINE, EMBASE, SciELO/LILACS, and Cochrane Library databases using the following key words (MeSH terms): Epworth, Berlin questionnaire, physical examination, body mass index, circumference, Mallampati, noise, pharynx, airway, Jaw, Diagnosis, Mass Screening, Diagnostic Techniques and Procedures, Diagnostic Tests, Laboratory Techniques and Procedures, Routine; Diagnostic Equipment/standards*; Comparative Effectiveness Research, Laryngoscopy, Cephalometry, Tomography, X-Ray Computed, Magnetic Resonance Imaging, Endoscopy, Pulmonary Ventilation, Polysomnography, Actigraphy, Sleep; Monitoring, Physiologic; Monitoring Sleep Apnea Syndromes, Sleep Disorders, Sleep Apnea, Obstructive; Sleep Initiation and Maintenance Disorders, Circadian Rhythm, Sleep, REM/physiology
*, Snoring, Disorders of Excessive Somnolence, Restless legs Syndrome, signs and symptoms, Fatigue, Headache, Delirium, Dementia, Amnestic, Cognitive Disorders, Mood Disorders, Fatigue Syndrome, Chronic; Questionnaires, survey Ambulatory, home care services, laboratory techniques and procedures, complications, adverse effects, Obesity, Overweight, Cardiovascular Diseases, Diabetes Mellitus, Stroke, Ischemic Attack, Transient; Gastroesophageal Reflux, Pulmonary Disease, Chronic Obstructive, Pre-Eclampsia, Pregnancy, Premature Birth, Post-menopause, Memory Disorders, Mental Disorders, Cognition Disorders, Neuropsychological Tests, Severity of Illness Index, Accidents, Traffic; Mortality.
Degree of recommendation and strength of evidenceA: Experimental or observational trials of higher consistency.
B: Experimental or observational trials of lesser consistency.
C: Case reports (non-controlled trials).
D: Opinions without critical evaluation, based on consensuses, physiological studies, or animal models.
ObjectiveTo evaluate the diagnoses of obstructive sleep apnea and primary snoring in adults and children, focusing on data from medical history, questionnaires, physical examination, and laboratory tests, as well as stimulating their investigation by general practitioners and several specialists.
INTRODUCTIONObstructive sleep apnea (OSA) is characterized by recurrent collapse of the pharynx during sleep, resulting in a substantial decrease in airflow (apnea or hypopnea). Respiratory events trigger intermittent disorders of blood gases (hypoxemia and hypercapnia) and can lead to sympathetic system activation.
Obstructive sleep apnea syndrome (OSAS) is associated with many symptoms and comorbidities, which include excessive daytime sleepiness, cognitive problems, obesity, type 2 diabetes mellitus, hypertension, exacerbation of chronic obstructive pulmonary disease (COPD), reduced quality of life, and significant increase in risk of industrial and traffic accidents. It is also considered an independent risk factor for cardiovascular disease and ischemic stroke.
Upper airway collapse during sleep is the result of an imbalance between the activity of pharyngeal dilator muscles and negative intraluminal pressure during inspiration. Factors that tend to narrow the pharynx lumen include mucosal adhesive forces, vasomotor tone, neck flexion, jaw opening and lower dislocation, force of gravity, increased nasal resistance, Bernoulli effect (the physics principle that explains the tendency of pharyngeal collapse), and increased dynamic compliance. Forces that dilate the pharynx include the thoracic caudal traction by increased pulmonary volume and neck extension.
Despite showing considerable variation between individuals, there are components of the disease physiopathology that have been already demonstrated, which include changes in the upper airway anatomy, variations in the capacity of the upper airway dilator muscles to respond to respiratory adversities during sleep, changes in cortical arousal threshold during an increase in inspiratory negative pressure, variations in the ventilatory control system stability, and changes in pulmonary volume.
OSAS is thought to be a progressive disease, and it is hypothesized that primary snoring and severe OSAS are opposite stages of the same disease. This pathological evolution would occur in the following chronological order: primary snoring, upper airway resistance syndrome, OSA, mild OSAS, moderate OSAS, and severe OSAS. Prompt diagnosis and appropriate treatment are important at any of these stages.
1. What is the clinical history of the patient with OSA? How important are questionnaires?The most frequent complaints of adult patients with OSA, when compared to non-apneic patients, are the presence of snoring, nocturnal choking, excessive daytime sleepiness (EDS), impotence, and nocturnal apneas reported by companions (p < 0.05)
1 (B). Other common symptoms include morning headaches, unrefreshing sleep, fatigue, and cognitive alterations.
Snoring and nocturia are common complaints in OSA
2 (B). Other clinical parameters such as body mass index (BMI), age, and gender are evaluated in Table 1. Men and women aged > 50 years diagnosed with OSA did not differ regarding the nature or severity of symptoms, as assessed by polysomnography (PSG) or the complaints of snoring, EDS, and perception of impaired diurnal function
3 (A).
Associating the subjective impression, which includes the clinical history, with physical examination and the PSG result of the apnea-hypopnea index (AHI) > 10 h allows for increased diagnostic certainty of OSA
1 (B).
To differentiate patients with and without apnea among snorers, the presence of OSAS (witnessed apnea, nocturnal choking, morning headache, or EDS) and alterations in the Epworth sleepiness scale (ESS), which must be
> 15 and BMI
> 28 kg/m
2, are assessed. The sensitivity to identify non-apneic individuals was 93.4% and the specificity was 60% (p < 0.001)
4 (B). This association of criteria is the best way to attain the clinical diagnosis of OSAS. When considering the disease prevalence of 15%, the presence of this association of criteria increases disease probability from 15% to 29% of cases, requiring complementary diagnostic confirmation by PSG.
Developed as a screening method for the detection of patients at high risk of OSA in primary care centers, the Berlin Questionnaire (BQ) (Box 1) has a sensitivity of 69% to 86% and specificity of 56% to 95% (positive predictive value of 77% to 96%)
5-9 (B). However, for the assessment of patients in sleep centers, it did not indicate favorable results due to high rates of false-positive and false-negative results, with a sensitivity of 61.5% to 62% and specificity of 22.6% to 43%, not allowing for an increase in diagnostic certainty10,11 (B). The validation of the Brazilian Portuguese version of the BQ in sleep centers identified 68.4% of the studied population as high-risk for OSA and 31.6% as low-risk. The sensitivity and specificity values of the BQ change in relation to AHI, but even in patients at high-risk for OSA, the altered BQ has a positive likelihood ratio (LR +) of 1.44 to 1.49
12 (A).
There is an association between alterations at the BQ in the population at high risk for OSA and patients with systemic arterial hypertension (SAH) resistant to clinical treatment
13 (B). Having SAH resistant to clinical treatment is a risk factor for OSA in the Brazilian population, with a sensitivity of 44% (31% to 58%), specificity of 91% (77% to 97%), increasing the diagnostic certainty of OSA from 15% to 46% (LR+ = 4.89; 95% CI: 2.52-9.47)
14 (A)
15 (B).
The ESS validated for Brazilian Portuguese16 (B) (Box 2), is very important in the identification of EDS (ESS > 10), assisting in the screening of patients with OSAS, mainly when associated with other clinical parameters
4,10,17,18 (B). Patients with ESS scores > 10 have a 2.5-fold higher risk of having OSA when compared with a normal test
17 (B). The prevalence of sleepiness (ESS > 10) increased with OSA severity, ranging from 21.4% (AHI < 5/h) to 40.2% (AHI > 30/h) (p < 0.001). However, less than half of patients with moderate to severe OSA reported somnolence (45.7%)19 (B). The scale has a sensitivity of 48% and specificity of 67%, providing a LR+ = 1.45 (1.03-2.06)
9 (B).
In children with sleep-disordered breathing (SDB), most associated symptoms are snoring, EDS, learning disorders, as well as somnambulism and somniloquy. Children with report of loud and frequent snoring have a 3.5-fold higher chance of having SDB, and children with EDS have a higher chance of presenting learning disorders and of male gender. The combination of the symptoms snoring or EDS with learning disorders has high specificity (97% and 98.9%, respectively), but low sensitivity (8.7% and 4.4%, respectively)
20 (B).
In preschoolers, the presence of snoring often or almost always has sensitivity of 64% and specificity of 57%
21 (B). Clinical evaluation has sensitivity of 68.4% and specificity of 59.5% for the diagnosis of OSA in children
22 (B).
Children with symptoms of SDB may have more EDS (OR = 2.2; 95% CI: 1.7-2.8) and behavioral problems, including hyperactivity (OR = 2.5; 95% CI: 2.0-3.0), attention deficit disorder (OR = 2.1; 95% CI: 1.7- 2.6), and aggression (OR = 2.1; 95% CI: 1.6 -2.6)
23 (B). They may also have alterations in growth, central auditory processing, and nocturnal enuresis
24,25 (C).
Table 1 compares the diagnostic values of different signs and symptoms suggestive of OSAS. The higher the positive likelihood ratio (LR+), the better. For example: a LR+ = 9 signifies that children with symptoms of SDB, EDS, and learning disorder have a nine-fold higher chance of having a confirmed diagnosis of OSA.
RecommendationThere is an increased likelihood of OSA diagnostic certainty in adults when the presence of symptoms is associated with alterations in ESS and increase in BM
I4 (B), whereas in young children the clinical diagnosis of OSA is associated with the presence of SDB symptoms, EDS, and learning disorders
20 (B), as highlighted by the a in Table 1, indicating a 2.3-fold and a nine-fold higher chance of OSA diagnosis, respectively.
The main symptoms of adult patients with OSA are snoring, nocturnal choking, EDS, impotence, and reporting of apneas by companions
1 (B). The combination of snoring with nocturia can be used in OSA screening
2 (B). There are differences between men and women older than 50 years regarding the nature and severity of symptoms
3 (A). Patients with SAH resistant to clinical treatment are more likely to have OSA; thus, they should always be assessed to rule out the disease
14 (A)
15 (B).
Children with SDB are more likely to have behavioral problems, including hyperactivity, attention deficit, aggression
23 (B), and enuresis
24,25 (C), as well as alterations in growth and central auditory processing
24,25 (C).
The Berlin Questionnaire (BQ) helps in the screening of patients at high risk of OSA in primary care centers
5-8 (B), but it does not allow for a definite diagnosis of OSA by itself
12 (A).
The ESS, along with other clinical parameters, helps to identify patients with OSA
4,10,17,18 (B). Although the prevalence of ESS > 10 increases with OSA severity, less than 50% of patients with moderate to severe OSA have ESS > 10
19 (B). The diagnostic contribution of the questionnaires is similar for both ESS and the BQ, with 1.45-
9 (B) and 1.44-1.49-fold
12 (A) higher chances of disease when the questionnaire results are altered, respectively.
2. What are the most important findings during physical examination of patients with OSA and primary snoring?The most relevant findings of the physical examination in adult patients with snoring/OSAS are obesity and alterations in the craniofacial skeleton and upper airways (UAs).
In addition to older age (> 50 years11)
26 (B) and male gender
26-28 (B), obesity markers, particularly increased BMI and neck circumference, are the main predictors of OSA presence
1,26-30 (B); however, the association between the degree of obesity and OSAS severity is still controversial
31,32 (B).
Using the AHI > 10/h, the prevalence of OSA in white men is 3.9%, while in women is 1.2%, maintaining statistically significant male:female ratio of 3.3:1 (p < 0.0006). This prevalence is modified when studying premenopausal women (0.6%) or postmenopausal women using hormone replacement therapy (0.5%); postmenopausal women with out hormone replacement present values that are similar to those in men (2.7%)
33 (A). In an epidemiological study conducted in the city of São Paulo, using clinical and polysomnographic criteria, the prevalence of OSA was 32.9% (95% CI: 29.6-36.3%), while maintaining independent associations in men (OR = 4.1; 95% CI 2.9-5.8), obese individuals (OR = 10.5; 95% CI: 7.1-15.7), and postmenopausal women (OR = 21; 95% CI: 1.4-3.9). There is an increase in OSA with increasing age, reaching an OR = 34.5 (95% CI: 18.5-64.2%) when compared with Brazilian groups aged 60-80 years to groups aged 20-29 years
30 (B).
BMI < 32.3 kg/m
2 was associated with OSAS of 0.4% (95% CI: 0.1-1.2), and BMI
> 32.3 kg/m
2 was associated with OSAS of 4.8% (95% CI: 2.5-9.0)
33 (A). When using BMI
> 32.3 kg/m
2 in the assessment of the physical examination of patients with snoring/OSA, a sensitivity of 92.5% (95% CI: 89.3%-95.8%) and specificity of 73.9% (95% CI: 61.2%-86.6%) were observed, increasing the LR+ from 1.5
32 (B) to 3.54
28 (B).
The neck circumference alone has a sensitivity of 60.6% (95% CI: 54.6 to 66.6%) and specificity of 93.4% (95% CI: 86.3%-100%), providing LR+ of 10.00 (95% CI: 4.53-22.07), increasing diagnostic probability from 15% to 64%
28 (B). The association of age > 50 years, neck circumference > 40 cm, and ESS > 10 increases the diagnostic certainty of OSA from 15% to 80% of cases. When applying the Kushida morphometric model to the Brazilian population, it was observed that the mean value of 36.7 cm (31 to 43 cm) can distinguish apneic from non-apneic individuals; in the sample studied, apneic individuals had a mean neck circumference of 40.4 cm (with a standard deviation of 4.1 cm), ranging from 31 to 54 cm
34 (B).
Through a morphometric model that associates BMI, neck circumference, and evaluation of craniofacial skeleton, considering a result found > 70, a sensitivity of 97.6% (95% CI: 95%-98.9%) and specificity of 100% (95% CI: 92%-100%) were observed. The morphometric model provides a LR+ = 97 (95% CI: 13.79-682). The use of this morphometric model should be encouraged, as it increases the likelihood of disease from 15% to 95% of cases
28 (B). In the Brazilian population, the morphometric model maintained the score > 70
34 (B).
Craniofacial alterations more often related to OSAS are those caused by maxillary and/or mandibular hypoplasia (Fig. 1), which can be identified by physical examination and confirmed by cephalometry
28,35,36 (B). In the Brazilian population, class II dental occlusion (retropositioned lower dental arch) was observed in 26.3% of cases, alterations in the hard palate (narrow or ogival) in 25.1% of cases, and mandibular hypoplasia in 19.7% of cases
36 (B) (Fig. 2).
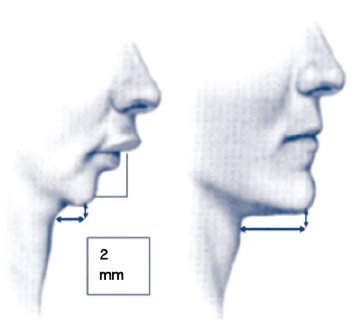
Figure 1 Retrognathia.
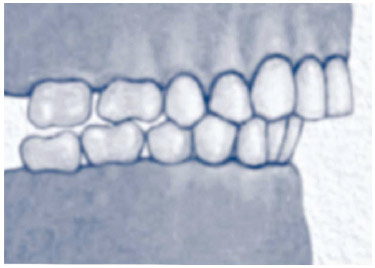
Figure 2 Class II dental occlusion (Angle).
Several anatomical alterations in the upper airways are described in patients with OSA. The most common findings are: nasal alterations; hyperplastic tonsils (Fig. 3); modified Mallampati index classes III and IV (Fig. 4) (inadequate association between the base the tongue and the oropharynx); and alterations in the soft palate, uvula, and tonsillar pillars
1,32,36,37 (B). In a Brazilian study, the most frequent findings in patients with OSA were the alterations in the soft palate (43.0%), modified Mallampati index classes III and IV (78.8%), abnormal tonsillar pillars (30.9%), uvular alterations (34.5%), septal deviations grade III (5.8%), and turbinate hypertrophy (49.8%)36 (B). The combination of BMI, modified Mallampati index, and presence of abnormal pharynx anatomy are related to OSA presence and severity in Brazilians
38 (B).
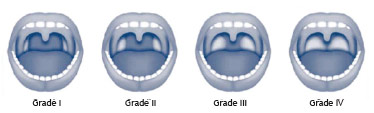
Figure 3 Grading system for palatine tonsils.
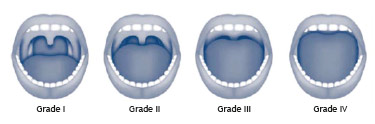
Figure 4 Modified Mallampati index.
Thus, it can be stated that patients with OSA are more obese and have higher values of neck circumference than control patients; however, the more obese patients do not always manifest more severe disease
31,32 (B). The prevalence of OSA in patients with class III obesity was shown to be greater than in the general population
32 (B). In a study in the Brazilian population, significant predictive factors for OSA in class III obese individuals were: mean age 44.6 ± 10.6 years and increased neck circumference, with a mean of 44.6 ± 5.2 cm
32 (B).
RecommendationOn physical examination of patients with snoring/OSA, the following factors must be taken into consideration: neck circumference measurements
28 (B), male gender
26-28 (B), (as there is a 3.3:1 ratio of men: pre-menopausal woman)
33 (A), older age (> 50 ± 11 years)
26 (B), and BMI values
28 (B). The most significant individual finding at the physical examination in patients with snoring/OSA is the neck circumference measure. The most relevant association in the physical examination includes BMI, neck circumference, and craniofacial skeleton assessment, called the morphometric model
2,28,34 (B). At the evaluation of the craniofacial skeleton, the anatomical alterations in the upper airways (UAs)
1,32,36,37 (B) and craniofacial abnormalities
28,35,36 (B) must be investigated. It is important to recall that patients with OSA are more obese, but the association between the degree of obesity and OSA severity is still controversial
31,32 (B).
3. When should PSG evaluation be indicated?PSG is a complementary test considered to be the gold standard, supporting the diagnosis and follow-up of OSAS
39 (B). Depending on the parameter alteration found during the examination, there will be different diagnostic probabilities, as described in Table 2. Overall, PSG provides a diagnostic certainty of 20% in a population with low prevalence of the disease (primary care with an estimated prevalence of 4%), while in the tertiary care population, with an estimated prevalence of 15%, the diagnostic certainty reaches 54% if AHI > 10
39,40 (B). The variability between nights of sleep can show conflicting results when the same patient is being monitored, which does not rule out the need for a new examination
41,42 (B). The AHI correlation between two PSG assessments in the same patient at 30-day intervals is poor (r = 0.44)
41 (B), and this variability of single-night PSG has an impact on diagnosis; in practice, approximately 13% of patients benefit from undergoing a second PSG assessment
42 (B). Since the PSG cannot confirm the diagnosis of OSA alone with AHI ranging from 5 to 15, it is necessary to associate the PSG results with the medical history and physical examination findings. Associating the subjective impression, which includes the medical history with physical examination and the PSG result of AHI > 10 h allows for an increase in the diagnostic certainty of AOS
1 (B). Considering the population with a low prevalence of the disease, this increases the likelihood from 4% to 28%, and when the population has a pretest prevalence of 15%, there is a 63% disease probability. In primary care, patients with neck circumference > 40 cm and PSG alterations will have an increase in the probability of disease from 20% to 71%. In tertiary care, patients with the same alteration in neck circumference and PSG alterations will demonstrate an increase in the probability of disease from 54% to 91%.
PSG can confirm the diagnosis of OSA alone when the AHI
> 15; however, this AHI value seldom appears alone, as it is often associated with BMI and neck circumference alterations
29 (B).
Epidemiological data including age range and gender show a higher prevalence of OSA in men with AHI > 15/h with OR = 2.7 (95% CI: 2.34-3.12)
29 (B) and age
> 50
26 (B).
Predictive factors obtained from the history and physical examination are suggestive of the presence of OSA, but the disease diagnosis with data concerning their intensity will only be attained by monitoring the patient's sleep, even in snoring patients
43 (B).
The presence of EDS investigated by ESS correlates with an increase in apneic episodes at the PSG (AHI < 5 in 21%, AHI > 5 in 28%, and AHI
> 30 in 35% of cases)
23 (B). A study that evaluated the ESS in 6,440 patients reported EDS (ESS > 10) in 1,149 patients (46%) with an AHI
> 15
19 (B).
The presence of snoring has been associated to the OSA diagnosis, presenting a sensitivity of 97.4%, specificity of 40%, positive predictive value of 82.3%, and negative predictive value of 84.2% for moderate to severe OSAS in a group with BMI > 25
44 (B). There is an association of EDS (ESS > 10) and frequent snoring (more than six nights a week) in patients with AHI > 15
45 (B).
In the presence of arterial hypertension, the correlation with severe OSA (AHI > 30) increased to 67% and, compared with patients with AHI < 15, an OR = 2.27 (95% CI: 1.76-2.92) was observed
46 (B). Another study demonstrated a correlation between severe OSAS in patients with BMI > 30
47 (B).
Obesity measured by BMI has been frequently associated with OSAS. Studies indicate that AHI worsens with increasing BMI
26,44 (B) and demonstrates an association of OSAS in patients with BMI > 35
48 (B).
RecommendationPSG should be indicated in patients with clinical suspicion of OSA and the presence of snoring
44,45 (B) associated or unassociated with EDS assessed by ESS
19,23 (B), neck circumference > 40 cm, obesity
26,44,48 (B), and arterial hypertension
46 (B), especially in the context of difficult-to-control hypertension
14 (A)
15 (B). The variability between nights of sleep sometimes requires the performance of a second PSG
41,42 (B).
The differential diagnosis between primary snoring and OSA can only be established after sleep monitoring
39,43 (B).
4. What are the sleep monitoring modalities and when should they be requested?There are four modalities of sleep monitoring:
Type I
Performed in a sleep laboratory with > seven channels for monitoringType II
Non-assisted with > seven channels for monitoringType III
Monitoring with four to seven channelsType IV
Monitoring with one or two channels, of which one is oximetry
The gold standard type-I PSG examination consists of the evaluation through at least seven channels to capture the physiological variables including electroencephalogram, electromyogram (chin and tibial), electrooculogram, airflow, respiratory effort, oxygen saturation, electrocardiogram, body position, and snoring. It is performed in a sleep laboratory, assisted by a PSG technician, with a minimum of six hours of monitoring; the data are interpreted by a physician qualified to interpret a report
39,49,51 (B)
52,53 (D).
Monitoring with portable equipment is classified by the number of capture channels available in each device. These tests may be assisted by a PSG technician, allowing for the examination to be performed in the patient's home
54,56 (D). A major limitation is the loss of monitoring channels due to failure, or loosening or disconnection of sensors, which has been estimated at between 4% and 33%, and the variability of equipment and technologies involved in the test
57 (D).
Portable sleep monitoring type II (PSM II) (comprehensive) comprises at least seven channels, including electroencephalogram, chin electromyogram, electrooculogram, airflow, respiratory effort, heart rate, and oxygen saturation. It allows for the identification of the different sleep stages with demonstration of statistics and calculations of AHI/h. Its limitation is the fact that it requires the technician to go to the patient's residence to set up the equipment and remove it on the following day, but if a channel is disconnected during the examination, there is no replacement
54-56 (D)
57 (D). PSM type II has shown similar results for AHI during at-home monitoring when compared to laboratory assessment
58 (B). It is estimated to have 70% of sensitivity and 91% of specificity
59 (B).
Portable monitoring type III (cardiopulmonary) uses between four and seven channels, including oxygen saturation, airflow, respiratory effort, and heart rate. It does not assess sleep stages and does not differentiate whether the events occur during the periods of wakefulness or sleep. It demonstrates and differentiates only respiratory events, not allowing for the diagnosis of other events, such as lower-limb movements. Some devices can be set up by the patient at home, without the need for a technician
55-57 (D)
58 (B). In a study of Brazilian patients, when indices were compared to type I monitoring, they showed results with strong correlation, with r = 0.876 (95% CI: 0.81-0.91; p < 0.0001) for any value of AHI (> 5, > 15, and > 30)
60 (A). Another equipment model presented similar results
61 (B).
Type IV monitoring uses one to two channels, and one of them must be oximetry. It does not assess sleep stages and does not differentiate between apnea types, but demonstrates desaturation. It does not allow for the evaluation of any data related to sleep
55-57 (D)
58 (B). In a study with Brazilian patients, similar results were observed when comparing the Type IV portable monitoring performed either in the sleep laboratory or at home
62 (A). Considering the high prevalence of OSA (33% in the population of São Paulo, SP, Brazil)
30 (B), type IV portable monitoring increases the disease likelihood to 57%
62 (A). Using the same type of equipment, studies have compared the rates of AHI with type 1 PSG and observed significant correlations, with r = 0.9563 (B) and r = 0.895
64 (B). Due to the fact that it is easy to repeat, three assessments were performed with a portable monitor on three consecutive nights, with no significant differences found between the values in these three examinations
63 (B).
The indication for type III and IV monitoring are still restricted to patients with high probability of OSA, whose assessment is based on anamnesis, physical examination, and questionnaires. If these types of monitoring do not diagnose OSA, type I or II monitoring is indicated to rule out a false negative result
56,57 (D).
PSG for titration of positive airway pressure (PAP) implies that the patient will return for a new sleep monitoring, assisted by technician in a sleep laboratory. The choice of treatment with PAP requires the identification of values at which the pressure produced by the machine can eliminate respiratory events. There is a protocol for the gradual increase in positive pressure associated with the placement of appropriate interfaces (mask). The correct PAP equipment to be used by the patient, with pressure identified by titration and the type of mask to be used, is indicated only after the titration
65,66 (D).
The choice of treatment with PAP means the indication of a long-term treatment. Measures of adherence to long-term treatment also depend on the type of titration to which the patient was submitted. The comparison of manual titration with automatic titration (both in the laboratory) has been discussed, but the results are still of short-term and without significant differences
67 (B). When comparing the two methods, it can be observed that both allow for improvements in AHI and sleepiness (ESS), with no differences in sleep architecture and treatment concordance, but with differences in treatment adherence
68 (B).
Split-night PSG in the first half of the night for the diagnosis and second half for PAP titulation. This modality does not allow for an accurate patient diagnosis, as it interrupts the evaluation halfway through the night and attempts to find the adequate pressure for the treatment in only half of the night. It should not be an elective procedure
53,65,66 (D).
The initial adherence to PAP comparing the titration of the whole night with the split-night shows similar results when comparing the number of days (78.7
vs. 77.5%), night hours of use (3.9
vs. 3.9 hrs), percentage of nights with use > four hours (52.9%
vs. 51.8%)
69 (B).
When performing a diagnostic investigation of OSA, the intention is to attain 75% or more of diagnostic certainty
70 (D). Considering that the association of clinical and supplementary examination still estimates the probability of disease between 25% and 75%, the investigation should be continued, adding other diagnostic methods.
The disease prevalence before the examination interferes with the diagnostic certainty in the presence of any altered results; Table 3 compares the diagnostic possibility among the four modalities of sleep monitoring, differentiating pre-testing low prevalence from high prevalence. There is a likelihood of OSA diagnostic certainty
> 75% when using only PSG I and PSG II in adult populations with high disease prevalence estimated at 32.9%
30 (B). Table 4 associates several diagnostic methods, such as signs and symptoms, physical examination, and two types of PSG (I and IV) in a population with low disease prevalence estimated at 4%
33 (A) in order to achieve diagnostic confirmation (diagnostic certainty > 75%
*). A male individual who snores does not demonstrate diagnostic certainty of OSA, even after PSG I is performed; similarly, a male obese individual does not demonstrate diagnostic certainty OSA, even after PSG I, as described in Table 4. In these two cases, before performing the first PSG, the neck circumference or the morphometric model should be investigated, as they both have a high positive likelihood ratio
28 (B), increasing diagnostic certainty. It may be necessary to perform the second PSG, due to AHI variability
41,42 (B). However, a thin male individual, younger than 50 years, with symptoms of OSA, neck circumference > 40 cm, and difficult-to-control hypertension is 76% certain to develop the disease, regardless of the PSG results (Table 4).
RecommendationMonitored full-night PSG performed in a sleep laboratory is considered the gold standard for OSAS diagnosis
39,49,51 (B). The diagnostic probability is similar when performing PSG I and II, as described in Table 3. Portable sleep monitoring assessments still have the limitation of monitoring channel loss due to failure, or loosening or disconnection of sensors
57 (D), and the need to perform a new PSG I or II to rule out false negatives in cases of high disease probability and normal initial monitoring results
56,57 (D).
The choice of treatment with PAP implies a monitored full-night PAP titration performed in a sleep laboratory by a PSG technician
65,66 (D). There are no significant differences in positive pressure tolerance time during the night, daytime sleepiness improvement, overall improvement, and patient satisfaction when comparing the whole-night PAP titration assisted by a technician with automatic titration
69-71 (B), but there are controversies regarding treatment adherence
67,68 (B).
5. When should PSG be requested in children?OSA in children has important conceptual, etiological, and classification differences when compared to adult apnea.
Snoring is a common complaint reported by the parents; however, the differentiation between primary snoring and OSA in children cannot be made solely based on clinical history data
21,72,73 (B). When comparing primary snoring with OSA, statistically different variations can be observed, such as daytime mouth breathing (61%
vs. 85%, p = 0.024), witnessed apnea (46%
vs. 74%, p = 0.013), and respiratory effort (58%
vs. 89 %, p = 0.003), but without enough strength to confirm the diagnosis of OSA
73 (B).
The presence of syndromic pictures, neuromuscular disease, and obesity are factors to be considered when requesting PSG assessment in children. Differentiation of pictures of central origin and estimation of apnea severity are important in the prevention of preoperative complications in children after tonsillectomy. Moreover, tonsillar size is not always a good indicative of the need for surgical intervention, and often, parents do not want to the child to undergo surgery and thus underestimate the child's symptoms
74 (D).
Table 6 shows the sensitivity, specificity, positive predictive value, and negative predictive values of the most common symptoms in children
75 (B).
Normal values for PSG in healthy children aged 1 to 15 years are: AI < 1.0 with maximum desaturation of 89%; expiratory PCO
2 cannot be > 45 mmHg for more than 10% of total sleep time
76 (B).
The American Academy of Sleep Medicine believes that the criteria of normality can be used up to the age of 18 years
77 (D). Studies have demonstrated that between 13 and 18 years, there is a difference when using the AHI criteria recommended for children and those recommended for adults, but this difference does not result in significant changes in the classification of OSA severity when the alternative criteria for adults are used
78 (B ).
The presence of OSA has increased and is recognized as a cause of morbidity, even in young children, with an estimated prevalence of 1% to 4%. Its diagnosis is important, as lack of treatment leads to learning and memory difficulties and decreased weight and height growth rates. In the long-term, it increases the risk of hypertension and depression
79 (D).
RecommendationPSG is recommended for all children with frequent snoring and who need to be differentiated from patients with OSA
21,72,73 (B). For the diagnosis of OSA, AI > 1, with saturation < 89% and/or expiratory PCO
2 > 45 mmHg for over 10% of total sleep time are considered diagnostic criteria
76 (B).
The AHI criteria established for children may be used up to the age of 18 years
77 (D). Between the ages of 13 and 18 years, the criteria recommended for children or the alternative criteria for adults may be used without change in OSAS classification
78 (B).
6. What is the importance of supplementary examinations in the investigation of OSA and snoring?There are other relevant, currently available tests for the evaluation of OSA and snoring, in addition to the gold standard (PSG). Among them are sleep endoscopy, video-nasofibrolaryngoscopy with Muller's maneuver, magnetic resonance imaging (MRI) and computed tomography (CT) of the upper airways, and cephalometry
80-90 (B).
Much has been discussed regarding the real importance of sleep endoscopy, which is the visualization of the upper airway through a flexible nasal endoscope during pharmacologically-induced sleep, to aid in the topographic diagnosis of snoring and OSA. When comparing patients with OSA using flexible video-nasofibrolaryngoscopy in wakefulness and during sleep endoscopy, aiming at the visualization of the pharyngeal obstruction site, similar results can be observed between the two tests in only 25% of cases. There was obstruction in the hypopharynx during pharmacologically-induced sleep in 33% of cases
80 (B).
Another study, comparing sleep endoscopy and video-nasofibroscopy with Muller's maneuver, showed that the surgical indication during wakefulness was 74%, whereas it was 54% at the sleep endoscopy analysis, corroborating the lack of agreement between examinations
81 (B). Patients with OSA who were using intraoral mandibular advancement devices underwent sleep endoscopy with and without the use of these devices. It was observed that patients who used the device (only those with successful treatments were evaluated) had a significantly increased upper airway area
82 (B).
When comparing the modified Mallampati index (MMI) of patients with primary snoring and OSA through sleep endoscopy, it was observed that there was no linear association between the obstruction level at the sleep endoscopy and the MMI. Patients with larger tongues (MMI 3 or 4) did not present narrowing at the region of the base of tongue; of this group, 76% had obstruction in the retropalatal region
83 (B).
Sleep endoscopy is not significantly relevant for the topographic diagnosis of OSA, which does not indicate irrelevance when assessing the apneic and snoring patient. It is worth mentioning that the criticism of this examination includes the fact that sleep is induced by medications, which can alter the pharyngeal muscle tone, lack of information regarding the patient's stage of sleep , and speculation on the pharyngeal region with non-segmented anatomical structure
80-83 (B).
Muller's maneuver is quite prevalent among otolaryngologists in the evaluation of patients with snoring and obstructive sleep apnea, but its real importance has been increasingly questioned. Comparing patients with a history of snoring and patients with OSA demonstrated by the PSG, it became clear that there are no significant differences between the two groups when analyzing BMI and retrolingual and retropalatal narrowing analyzed by video-nasofibrolaryngoscopy with Muller's maneuver. However, a positive association between BMI, AHI, and retrolingual obstruction is observed when considering only the group of patients with apnea
84 (B). Video-nasofibrolaryngoscopy with Muller's maneuver was performed in the supine and standing positions in OSAS patients, scanning the images on specific software to minimize the subjectivity of evaluation, subsequently comparing the results with MRI of the pharyngeal region. There is an agreement between the two methods of 93.3% in the retropalatal and of 95.6% in the retrolingual region
85 (B). Thus, it can be observed that Muller's maneuvers do not have the capacity to significantly alter management in patients with OSA, especially because the patient is usually evaluated in a non-supine position and during wakefulness. Furthermore, it is noteworthy that there is subjectivity and lack of homogeneity during this assessment, as the inspiratory force which the patients present varies significantly and evaluation data are yet to be established.
Imaging tests such as MRI, CT, and cephalometry are non-invasive and can be objective in their results; however, their actual relevance continues to prompt studies in several reference centers in the world.
When evaluating patients with OSA and normal patients through MRI of the upper airway, no significant differences were observed between the two groups regarding the internal distance between the two mandibular condyles and the mandible bone thickness. However, patients in the apnea group have greater mandibular discrepancy, a smaller internal mandibular length, and a smaller area in the mandibular basal plane than the control group. There were no significant differences in the morphological parameters of the mandible between the obese and nonobese patients with apnea. The volumes of the tongue, soft palate, and lateral pharyngeal walls did not differ significantly between the groups
86 (B). Ultrafast MRI (0.8 s) was performed in patients with OSAS (AHI > 10) during wakefulness and sleep, and in non-apneic patients (through clinical history and nocturnal oximetry) during wakefulness. It was observed that, during part of the respiratory cycle, the velopharyngeal region is smaller in apneic patients, and that the variation in the velopharyngeal area during the respiratory cycle is greater in apneic patients, particularly during sleep, suggesting a greater plasticity of the upper airway in these patients. Furthermore, they verified that the area of pharyngeal narrowing was similar in both the anteroposterior and latero-lateral views, both in controls and in apneic individuals during wakefulness; however, during sleep, apneic individuals have maximal circular narrowing of the pharynx. There is also an inverse association between dimensions of the lateral pharyngeal walls and the airway area, probably indicating that the lateral walls are passively compliant as a result of changes in airway caliber. It was observed that the volume of the soft palate and adipose tissue in the parapharyngeal region is higher in apneic patients
87 (B).
Focusing on the upper airway CT in the evaluation of patients with sleep-disordered breathing, this imaging study was evaluated in patients with apnea (AHI > 5) and 24 primary snorers (AHI < 5) with oro- and hypopharynx measurements, and correlated with the obstructive apnea severity indices and cephalometric studies. Patients with severe OSA had significantly greater narrowing in the uvula region during expiration, more inferiorly positioned hyoid bone, larger volume of soft palate, and larger neck circumference when compared with primary snorers and patients with mild to moderate OSA
88 (B). When comparing the CT and PSG in patients with obstructive sleep-disordered breathing (1/6 with primary snoring and 5/6 with OSAS), a significant association between retropalatal and latero-lateral pharyngeal diameters with high rates of AHI was observed. It was also verified that retropalatal and retroglossal spaces were predictive of the apnea index severity. None of the CT parameters correlated with intensity of snoring and minimal O
2 saturation. BMI correlated positively and significantly with retropalatal distance and AHI. From the anatomical standpoint, the latero-lateral retropalatal view is significantly associated with impairment of the upper airway caliber in patients with sleep-disordered breathing
89 (B). When comparing the upper airway diameter by CT in patients with OSAS and healthy patients, no correlation existed between PSG parameters in obstructive sleep-disordered breathing (minimum O
2 saturation and AHI) and pharyngeal dimensions
90 (B). There were no significant differences between the tomographic measurements of the pharyngeal airway in vigil patients submitted to lateral pharyngoplasty compared to uvulopalatopharyngoplasty, although there were differences in clinical and polysomnographic results of this population; therefore, there was no correlation between polysomnographic and CT parameters in OSAS patients submitted to surgery
91 (B). Areas of the nasopharynx, oropharynx, and hypopharynx were evaluated during inspiration and expiration, as well as the diameters of the uvula and retropharyngeal tissue to compare the results obtained by CT in patients with apnea (AHI > 10) and without apnea (AHI < 10). It was observed that the retropharyngeal tissue in apneic patients presents more volume than in non-apneic patients, with 10.3 ± 3.6 mm
vs. 6.4 ± 2.7 mm, p < 0.01. However, the nasopharynx areas during expiration (228.4
vs. 281.9 mm) and inspiration (195.9
vs. 300.4 mm) of the non-apneic patients were slightly larger, but without statistically significant differences
91 (B).
When studying patients with OSAS and controls by PSG and TC, in which OSAS patients had undergone uvulopalatopharyngoplasty, it was observed that severely apneic patients had a narrower sectional area of the oropharynx (50 mm
2 on average) when compared to the others. The control patients and patients submitted to uvulopalatopharyngoplasty without OSAS, i.e., with primary snoring, had minimal pharyngeal area of 110 mm
2, on average. Furthermore, patients with moderate OSAS submitted to surgery demonstrated values between 60 and 100 mm
2 (B).
91Cephalometry is a useful method in the evaluation of apneic and snoring patients
93 (C). The choice to request this examination as a routine investigation in a patient with OSA has been the subject of debate, since it has not been proved that it actually changes the therapeutic approach. However, it can be observed that cephalometry is crucial in the surgical planning of patients submitted to orthognathic surgery for the treatment of OSAS. The association between cephalometry and the degree of OSAS severity in adult patients with and without obstructive sleep apnea was analyzed. It was observed that the length of the upper airway was strongly correlated with the severity of OSA in men (r = 0.72, p < 0.01) and moderately associated in women with OSAS (r = 0.52, p < 0.01)
94 (C).
Thus, imaging tests, even during induced sleep, require further studies to assess the applicability of their results as auxiliary examinations when defining conducts. Currently, these tests are more relevant for the development of research in this area.
RecommendationSleep endoscopy is employed in the clinical investigation of apneic and snoring patients, but controversies still exist regarding its applicability in routine assessment
80-83 (B).
Flexible video-nasofibrolaryngoscopy does not change the conduct in patients with snoring and OSA, and there is no homogeneity of results among different observers
84,85 (B).
MRI and CT of the pharynx are noninvasive tests and demonstrate that apneic patients have a narrower sectional pharynx area than non-snorers and non-apneic patients, but the location of these strictures varies between individuals
87,88,90 (B).
Cephalometry is altered in apneic patients when compared to non-apneic patients, and it is essential for surgical planning in patients who will undergo orthognathic surgeries
92,93 (C).
7. What are the consequences of OSAS?Moderate to severe OSAS is an independent predictor of all-cause mortality, with HR = 6.24 (95% CI: 2.01-19.39)
95 (A), and this association is not attributable to obesity, age, or other medical chronic conditions, especially in men with severe OSAS aged 40 to 70 years
95 (A). Mild OSAS is not an independent risk factor for all-cause mortality, with HR = 0.47 (95% CI: 0.17-1.29)
95 (A). An increased risk of coronary events or death from cardiovascular causes can be observed, regardless of other factors, in patients aged > 50 years
95 (A)
96,97 (B), with HR = 2.06 (95% CI: 1.10-3.86)
98 (A). A self-assessment by standardized questionnaire showed a greater association between OSAS and heart failure and stroke than with coronary heart disease
96 (B).
In healthy middle-aged adults and in the elderly, OSAS is associated with increased prevalence of systemic arterial hypertension. Correcting for the main confounding factors (age, gender, BMI, and other measures of adiposity), as well as other potentially relevant variables (tobacco and alcohol consumption), higher AHI and longer desaturation time < 90% were associated with higher risk of hypertension, with OR = 1.37 (95% CI: 1.03-1.83)
99 (B). Reduction in systolic dipping related to OSA severity was demonstrated: when AHI < 5, hypertension had OR = 3.1 (95% CI: 1.3-7.7), but when AHI > 15, hypertension had OR = 4.4 (95% CI: 1.2-16.31). This lack of systolic dipping can be one of the mechanisms by which OSA contributes to an increase in cardiovascular diseases
100 (B).
There is evidence that patients with OSA older than 40 years without underlying arterial hypertension may develop the disease perhaps partly due to the influence of obesity; however, in patients with AHI > 30, a small influence of OSA itself cannot be ruled out in the genesis of hyperten
sion46,47,99
(B).OSAS is an independent risk factor for developing type II diabetes
101 (A)
102 (B)
103 (C), with HR = 1.4 (95% CI: 1.10-1.86), p = 0.008
101 (A).
Reduced time of sleep is associated with obesity in adults. Sleep lasting less than five hours per night is associated with central obesity and increased body fat percentage and body mass index, on average 2.5 kg/m
2 (95% CI: 2.0-2.9) for men and 1.8 kg/m
2 (95% CI: 1.1-2.4) for women
104 (B). Patients with OSAS and metabolic syndrome103 (C) and/or morbid obesity
105 (B) have increased sympathetic tone, with increased cardiovascular risk. Children and adolescents with OSAS and a BMI percentile > 85% assessed by questionnaires have lower quality of life
106 (B).
OSAS is an independent risk factor for ischemic stroke
95,107 (A), with a relative risk of death from stroke (RR) = 5.16 (95% CI: 3.72- 6.60)
108 (B). The risk is higher for men with mild to moderate OSAS
109 (B), with HR = 6 (95% CI: 2%-10%)110 (A). Menopausal women, aged 50-79 years, of whom 8.3% had sleep duration < five hours per night and 4.6% had nine hours of sleep per night, were followed-up on average for 7.5 years. There is an association between long sleep duration and risk of ischemic stroke, with RR = 1.70 (95% CI: 1.32-2.21) for those who slept nine hours a night, regardless of the presence of snoring or somnolence. There was no significant association between ischemic stroke and short sleep duration
111 (A). A reduction in daytime sleepiness and BMI was verified in patients after ischemic stroke; this population may be underdiagnosed for OSAS
95 (A).
Untreated OSAS is a contributing factor in motor vehicle accidents
112,113 (B). Treatment of OSAS with CPAP reduces the relative risk of collisions, with RR = 0.278 (95% CI: 0.22- 0.35), p < 0.001
112 (B).
Sleep apnea is associated with a higher prevalence of psychiatric comorbidities such as depression (21.8%), anxiety disorder (16.7%), post-traumatic stress disorder (11.9%), and psychosis and bipolar disorder (3.3%)
114 (B).
Several studies have proposed an association between OSAS and neurocognitive dysfunction
115,116 (B). When assessing elderly individuals considered healthy for their age, with a mean age of 68 years, 58.5% females, the performance of the PSG showed that 53% had an AHI > 15 events/h and 37% had AHI > 30 events/h, of whom only 9.2% had EDS (ESS > 10). There was no significant association between AHI, nocturnal hypoxemia, and cognitive performance, except a tendency toward slower performance in patients with AHI > 30 events/h
115 (B). Mild to moderate OSAS has minimal impact on measures related to attention or speed of executive functions and processing speed when comparing OSAS individuals with the non-apneic
117 (B). OSAS may exacerbate cognitive impairment in dementia caused by Alzheimer's disease
118 (B).
Gastroesophageal reflux disease (GERD) is more prevalent in symptomatic patients with sleep-disordered breathing; however, the occurrence of gastroesophageal reflux or reflux symptoms was not significantly influenced by the severity of OSAS. There appears to be an increasing index of microarousal caused by reflux in patients with OSAS when compared to the simple snorer
119 (B).
Patients with chronic obstructive pulmonary disease (COPD) and OSAS ("overlap syndrome") have an increased risk of death and hospitalization for COPD exacerbation. At a mean follow-up of 9.4 years (3.3 to 12.7 years) patients with overlap syndrome not treated with CPAP had higher mortality when compared to those using CPAP, with RR = 1.79 (95% CI: 1.16-2.77) and increased tendency to COPD exacerbation requiring hospitalization, with RR = 1.70 (95% CI: 1.21-2.38)
120 (B).
In obese OSA patients without COPD, daytime hypercapnia is associated with OSAS severity, higher BMI, and higher mechanical restriction of the chest wall
121 (B).
Pregnant women with OSAS have higher risk of pre-eclampsia, premature birth, and medical complications
122 (C). For pregnant women with chronic snoring and hypertension, the use of nasal CPAP, while maintaining the usual prenatal care treatment, appears to improve blood pressure and prevent complications
123 (B).
RecommendationModerate to severe OSAS is a risk factor for mortality from any cause
95 (A). OSAS increases the risk of coronary artery disease
98 (A), type 2 diabetes mellitus
101 (A), ischemic stroke
110 (A), hypertension
47,99,100 (B), obesity
104 (B), hospitalization, and death from COPD exacerbation
120 (B). There is an association of OSAS with psychiatric comorbidities
114 (B), preeclampsia and premature birth
123 (B), in addition to contributing to automobile accidents
112,113 (B). Considering the severe consequences of OSAS, it should always be investigated to prevent underdiagnosis and treatment delay.
REFERENCES1. Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16:118-22.
2. Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010;14:337-43.
3. Bailes S, Baltzan M, Alapin I, Fichten CS, Libman E. Diagnostic indicators of sleep apnea in older women and men: a prospective study. J Psychosom Res. 2005;59:365-73.
4. Lim PV, Curry AR. The role of history, Epworth Sleepiness Scale Score and body mass index in identifying nonapnoeic snorers. Clin Otolaryngol Allied Sci. 2000;25:244-8.
5. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423-38.
6. Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124:281-90.
7. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485-91.
8. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822-30.
9. Olson LG, Cole MF, Ambrogetti A. Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J Sleep Res. 1998;7:248-53.
10. Friedman M, Wilson MN, Pulver T, Pandya H, Joseph NJ, Lin HC, et al. Screening for obstructive sleep apnea/hypopnea syndrome: subjective and objective factors. Otolaryngol Head Neck Surg. 2010;142:531-5.
11. Ahmadi N, Chung SA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:39-45.
12. Vaz AP, Drummond M, Mota PC, Severo M, Almeida J, Winck JC. Translation of Berlin Questionnaire to Portuguese language and its application in OSA identification in a sleep disordered breathing clinic. Rev Port Pneumol. 2011;17:59-65.
13. Gus M, Gonçalves SC, Martinez D, de Abreu Silva EO, Moreira LB, Fuchs SC, et al. Risk for Obstructive Sleep Apnea by Berlin Questionnaire, but not day time sleepiness, is associated with resistant hypertension: a case-control study. Am J Hypertens. 2008;21:832-5.
14. Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811-7.
15. Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135-9.
16. Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Menna Barreto SS, Johns MW. Portuguese-language version of the Epworth sleepiness scale: validation for usein Brazil. J Bras Pneumol. 2009;35:877-83.
17. Santaolalla Montoya F, Iriondo Bedialauneta JR, Aguirre Larracoechea U, Martinez Ibargüen A, Sanchez Del Rey A, Sanchez Fernandez JM. The predictivevalue of clinical and epidemiological parameters in the identification ofpatients with obstructive sleep apnoea (OSA): a clinical prediction algorithm in the evaluation of OSA. Eur Arch Otorhinolaryngol. 2007;264:637-43.
18. Subramanian S, Hesselbacher SE, Aguilar R, Surani SR. The NAMES assessment: a novel combined-modality screening tool for obstructive sleep apnea. Sleep Breath. 2011;15:819-26.
19. Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28:472-7.
20. Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing-the Tucson Children's Assessment of Sleep Apnea study (TuCASA). J Clin Sleep Med. 2005;1:247-54.
21. Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27:87-94.
22. Sproson EL, Hogan AM, Hill CM. Accuracy of clinical assessment of paediatric obstructive sleep apnoea in two English centres. J Laryngol Otol. 2009;123:1002-9.
23. Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502-7.
24. Goldstein NA, Post JC, Rosenfeld RM, Campbell TF. Impact of tonsillectomy and adenoidectomy on child behavior. Arch Otolaryngol Head Neck Surg. 2000;126:494-8.
25. Ziliotto KN, dos Santos MF, Monteiro VG, Pradella-Hallinan M, Moreira GA, Pereira LD, et al. Auditory processing assessment in children with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2006;72:321-7.
26. Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med. 1991;115:356-9.
27. Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47:101-5.
28. Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127(8 Pt 1):581-7.
29. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community- dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893-900.
30. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441-6.
31. Fogel RB, Malhotra A, Dalagiorgou G, Robinson MK, Jakab M, Kikinis R, et al. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep. 2003;26:150-5.
32. Martinho FL, Tangerina RP, Moura SM, Gregorio LC, Tufik S, Bittencourt LR. Systematic head and neck physical examination as a predictor of obstructive sleep apnea in class III obese patients. Braz J Med Biol Res. 2008;41:1093-7.
33. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144-8.
34. Soares MC, de Azeredo Bittencourt LR, Zonato AI, Gregório LC. Application of the Kushida morphometric model in patients with sleep-disordered breathing. Braz J Otorhinolaryngol. 2006;72:541-8.
35. Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427-32.
36. Zonato AI, Martinho FL, Bittencourt LR, de Oliveira Campones Brasil O, Gregorio LC, Tufik S. Head and neck physical examination: comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope. 2005;115:1030-4.
37. Friedman M, Tanyeri H, La Rosa M, Landsberg R, Vaidyanathan K, Pieri S, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109:1901-7.
38. Zonato AI, Bittencourt LR, Martinho FL, Júnior JF, Gregório LC, Tufik S. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2003;113:973-80.
39. Ross SD, Sheinhait MA, Harrison Kl, Kvasz M, Connelly JE, Shea AS, et al. Systematic Review and Meta-analysis of the literature Regarding the Diagnosis of Sleep Apnea. Sleep. 2000;23:1-14.
40. Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279-85.
41. Levendowsky DJ, Zack N, Rao S, Wong K, Gendreu M, Kranzler J, et al. Assesment of the test-retest reliability of laboratory polysomnography. Sleep Breath. 2009;13:163-7.
42. Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13:211-26.
43. Dreher A, Chaux R, Klemens C, Werner R, Baker F, Barthlen G, et al. Correlation Between Otorhinolaryngologic Evaluation and Severity of Obstructive Sleep Apnea Syndrome in Snorers. Arch Otolaryngol Head Neck Surg. 2005;131:95-98.
44. Morris LG, Kleinberger A, Lee KC, Liberatore LA, Burschtin O. Rapid risk stratification for obstructive sleep apnea,based on snoring severity and body mass index. Otol Head Neck Surg. 2008;139:615-8.
45. Gottlieb DJ, Yao Q, Redeline S, Ali T, Mahowald MW. Does Snoring Predict Sleepiness Independently of Apnea and Hypopnea Frequency? Am J Respir Crit Care Med. 2000;162:1512-7.
46. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of Sleep Disordered Breathing, Sleep Apnea and Hypertension in a Large Commnunity Based Study. JAMA. 2000;283:1829-36.
47. Bixler EO, Vgontzas AN, Lin HM, Have TT, Leiby BE, Vela-Bueno A, et al. Association of Hypertension and Sleep-Disordered Breathing. Arch Intern Med. 2000;160:2289-95.
48. Dixon JB, Schachter LM, O'Brien PE. Predicting Sleep Apnea and Excessive Day Sleppiness in the Severe Obese: Indicators for Polysomnography. Chest. 2003;123:1134-41.
49. Andreas S, von Breska B, Magnusson K, Kreuzer H. Validation of automated sleep stage and apnoea analysis in suspected obstructive sleep apnoea. Eur Respir J. 1993;6:48-52.
50. Khawaja IS, Olson EJ, van der Walt C, Bukartyk J, Somers V, Dierkhising R, et al. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med. 2010;6:357-62.
51. Ross SD, Sheinhait IA, Harrison KJ, Kvasz M, Connelly JE, Shea SA, et al. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23:519-32.
52. Chesson AL Jr, Ferber RA, Fry JM, Grigg-Damberger M, Hartse KM, Hurwitz TD, et al. The indications for polysomnography and related procedures. The indications for polysomnography and related procedures. Sleep. 1997;20:423-87.
53. Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499-521.
54. Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17:372-7.
55. Ferber R, Millman R, Coppola M, Fleetham J, Murray CF, Iber C, et al. Portable Recording in the Assessment of Obstructive Sleep Apnea. Sleep. 1994;17:378-92.
56. Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive. Sleep. 2007;3:737-47.
57. Kuna ST. Portable-Monitor Testing: An Alternative Strategy for Managing Patients With Obstructive Sleep Apnea. Respiratory Care. 2010;55:1196-215.
58. Iber C, Redline S, Glipin AM, Quan SF, Zhang L, Gottlieb DJ, et al. Polysomnography performed in the unattend home versus attended laboratory setting - sleep heart health study methodology. Sleep. 2004;27:536-40.
59. Flemons WW, Littner MR, Rowley JA, Gay P, Anderson WM, Hudgel DW, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543-79.
60. Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629-36.
61. Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS). Respirology. 2010;15:336-42.
62. Tonelli de Oliveira AC, Martinez D, Vasconcelos LF, Gonçalves SC, Lenz MC, Fuchs SC, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135:330-6.
63. Ragette R, Wang Y, Weinreich G, Teschler H. Diagnostic performance of single airflow channel recording (ApneaLink) in home diagnosis of sleep apnea. Sleep Breath. 2010;14:109-14.
64. Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnea syndrome. Intern Med J. 2009;39:757-62.
65. Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157-71.
66. Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375-80.
67. Galetke W, Rnaderath WJ, Stieglitz S, Laumanns C, Anduleit N, Richter K, et al. Comparison of Manual titration and automatic titration based on force oscillation technique, flow and snoring in obstructive sleep apnea. Sleep Medicine. 2009;10:337-43.
68. Gao W, Jin Y, Wang Y, Sun M, Chen B, Zhou N, et al. Is automatic CPAP titration as effective as manual CPAP titration in OSAHS patients? A meta-analysis. Sleep Breath. 2012;16:329-40.
69. Collen J, Holley A, Lettieri C, Shah A, Roop S. The impact of split-night versus traditional sleep studies on CPAP compliance. Sleep Breath. 2010;14:93-9.
70. Nobre M, Bernardo WM. Prática clínica baseada em evidências. Rio de Janeiro: Elsevier, 2006, p. 115.
71. Kuna ST, Gurubhagavatula I, Maislin G, Hin S, Hartwig KC, McCloskey S, et al. Noninferiority of functional outcome ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238-44.
72. Lamm C, Mandeli J, Kattan M. Evaluation of home audiotapes as an abbreviated test for obstructive sleep apnea syndrome (OSAS) in children. Pediatr Pulmonol. 1999;27:267-72.
73. Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610-8.
74. Roland PS, Rosenfeld RM, Brooks LJ, Friedman NR, Jones J, Kim TW, et al. Clinical practice huideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol HNS. 2011;145:S1
75. Saeed MM, Keens TG, Stabile MW, Bolokowicz J, Davidson Ward SL. Should children with suspected obstructive sleep apnea syndrome and normal nap sleep studies have overnight sleep studies? Chest. 2000;118:360-5.
76. Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872-8.
77. Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169-200.
78. Accardo JA, Shults J, Leonard MB, Traylor J, Marcus CL. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep. 2010;33:1333-9.
79. Sinha D, Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131:311-20.
80. Campanini A, Canzi P, De Vito A, Dallan I, Montevecchi F, Vicini C. Awake versus sleep endoscopy: personal experience in 250 OSAHS patients. Acta Otorhinolaryngol Ital. 2010;30:73-7.
81. Hewitt RJ, Dasgupta A, Singh A, Dutta C, Kotecha BT. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol. 2009;266:691-7.
82. Johal A, Battagel JM, Kotecha BT. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with mandibular advancement splints in obstructive sleep apnoea. Eur J Orthod. 2005;27:607-14.
83. den Herder C, van Tinteren H, de Vries N. Sleep endoscopy versus modified Mallampati score in sleep apnea and snoring. Laryngoscope. 2005;115:735-9.
84. Ko MT, Su CY. Computer-assisted quantitative evaluation of obstructive sleep apnea using digitalized endoscopic imaging with Muller maneuver. Laryngoscope. 2008;118:909-14.
85. Hsu PP, Tan BY, Chan YH, Tay HN, Lu PK, Blair RL. Clinical predictors in obstructive sleep apnea patients with computer-assisted quantitative videoendoscopic upper airway analysis. Laryngoscope. 2004;114:791-9.
86. Okubo M, Suzuki M, Horiuchi A, Okabe S, Ikeda K, Higano S, et al. Morphologic analyses of mandible and upper airway soft tissue by MRI of patients with obstructive sleep apnea hypopnea syndrome. Sleep. 2006;29:909-15.
87. Ciscar MA, Juan G, Martínez V, Ramón M, Lloret T, Mínguez J, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79-86.
88. Yucel A, Unlu M, Haktanir A, Acar M, Fidan F. Evaluation of the upper airway cross-sectional area changes in different degrees of severity of obstructive sleep apnea syndrome: cephalometric and dynamic CT study. AJNR Am J Neuroradiol. 2005;26:2624-9.
89. Li HY, Chen NH, Wang CR, Shu YH, Wang PC. Use of 3-dimensional computed tomography scan to evaluate upper airway patency for patients undergoing sleep-disordered breathing surgery. Otolaryngol Head Neck Surg. 2003;129:336-42.
90. Bhattacharyya N, Blake SP, Fried MP. Assessment of the airway in obstructive sleep apnea syndrome with 3-dimensional airway computed tomography. Otolaryngol Head Neck Surg. 2000;123:444-9.
91. Cahali MB, Formigoni GG, Gebrim EM, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27:942-50.
92. Caballero P, Alvarez-Sala R, García-Río F, Prados C, Hernán MA, Villamor J, et al. CT in the evaluation of the upper airway in healthy subjects and in patients with obstructive sleep apnea syndrome. Chest. 1998;11:111-6.
93. Avrahami E, Englender M. Relation between CT axial crosssectional area of the oropharynx and obstructive sleep apnea syndrome in adults. AJNR Am J Neuroradiol. 1995;16:135-40.
94. Susarla SM, Abramson ZR, Dodson TB, Kaban LB. Cephalometric measurement of upper airway length correlates with the presence and severity of obstructive sleep apnea. J Oral Maxillofac Surg. 2010;68:2846-55.
95. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079-85.
96. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19-25.
97. Endeshaw YW, Unruh ML, Kutner M, Newman AB, Bliwise DL. Sleep-disordered breathing and frailty in the Cardiovascular Health Study Cohort. Am J Epidemiol. 2009;170:193-202.
98. Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131-6.
99. O'Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159-64.
100. Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31:795-800.
101. Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122-7.
102. Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15-20.
103. Trombetta IC, Somers VK, Maki-Nunes C, Drager LF, Toschi-Dias E, Alves MJ, et al. Consequences of comorbid sleep apnea in the metabolic syndrome-implications for cardiovascular risk. Sleep. 2010;33:1193-9.
104. Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-34.
105. Vanhecke TE, Franklin BA, Zalesin KC, Sangal RB, de Jong AT, Agrawal V, et al. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134:539-45.
106. Carno MA, Ellis E, Anson E, Kraus R, Black J, Short R, et al. Symptoms of sleep apnea and polysomnography as predictors of poor quality of life in overweight children and adolescents. J Pediatr Psychol. 2008;33:269-78.
107. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132.
108. Capampangan DJ, Wellik KE, Parish JM, Aguilar MI, Snyder CR, Wingerchuk D, et al. Is obstructive sleep apnea an independent risk factor for stroke? A critically appraised topic. Neurologist. 2010;16:269-73.
109. Parra O, Arboix A, Montserrat JM, Quintó L, Bechich S, García-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:267-72.
110. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269-77.
111. Chen JC, Brunner RL, Ren H, Wassertheil-Smoller S, Larson JC, Levine DW, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185-92.
112. Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motorvehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373-80.
113. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573-81.
114. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405-11.
115. Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515-21.
116. Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-81.
117. Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498-507.
118. Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5:305-9.
119. Valipour A, Makker HK, Hardy R, Emegbo S, Toma T, Spiro SG. Symptomatic gastroesophageal reflux in subjectis with a breating sleep disorder. Chest. 2002;121:1748-53.
120. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325-31.
121. Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest. 2009;136:787-96.
122. Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261.e1-5.
123. Poyares D, Guilleminault C, Hachul H, Fujita L, Takaoka S, Tufik S, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15-21.
* The Guidelines Project is a joint initiative of the Brazilian Medical Association and the Federal Council of Medicine, aiming to compile information from the medical area in order to standardize procedures to assist in the rationale and decision-making of physicians. The information contained in this project must be submitted for the assessment and analysis of the physician in charge of treatment, considering the reality and clinical status of each patient.


