INTRODUCTIONRecurrent aphthous stomatitis (RAS) is a common disease that attacks the oral mucosa and affects over 10% of the world population28, 42, 43. The evidence points to the fact that RAS is associated to immune-mediated reactions41, 42. The evidence and the incidence of family RAS increase the chances of the affection be associated to HLA-system antigens41. Lesions are classified into three groups: minor aphthous, major aphthous and herpes ulcers41, 43. Histopathology of the oral lesions reveals infiltrate of mononuclear cells of pre-ulcer stage lesions. In ulcerated forms there is an accumulation of polymorphonuclear leukocytes. A great number of mast cells were found in early RAS lesions. Their presence is reduced in more advanced stages of the disease probably because of their degranulation or destruction42. The affection is occasionally present after the intake of some types of foods and IgE-releasing lymphocytes that are increased in oral lesions and peripheral blood. IgG and IgM serum levels can be unaffected and IgA may be increased42. The final diagnosis occurs between 3 weeks and 1 year because it is usually diagnosed as oral candidiasis, trauma lesions or herpes ulcers1.
ConceptRAS is a chronic oral mucosa affection of common occurrence and it is characterized by onset of ulcer lesions in any region of the jugal mucosa28, 29, 35, 41. These lesions can vary in size, quantity and location. Normally they resolve spontaneously and may have a recurrent character39, 41. Its etiology is multifactorial and they are associated to local origin causes, such as traumas, or systemic causes, such as infections and immunohematological diseases.
HistoryIt is believed that Hyppocrates was the first one to use the word "aphtai" to describe lesions present in the oral mucosa probably referring to mycotic stomatitis lesions39. Mikulicz and Kümmel were the first authors to publish a clinical description about RAS in 1988. In 1899, Sibley described what we call today minor aphthous ulcer and attributed its cause to stress and psychical states, naming it "neurotic ulcer"39. The first report of major aphthous ulcer was made by Sutton in 1911. The author used the term "periadenitis mucosa necrotica recurrens", but his studies referred to deep ganglia only, which are rarely affected. For this reason, the name was not accepted by other authors. The current designation was given by Cooke and Lehner39. The same Cooke in 1960 described for the first time the herpetiform ulcers, a term used owing to the similarity between the lesion caused by the infection with herpes simplex. Despite the various studies, the author was unable to prove the viral etiopathogenesis of RAS, even using cytological, serologic and culture tests and histopathology as support.39
Epidemiology
RAS is a very common affection. It affects 5 to 66% of the world population, ranging in incidence according to the social and population characteristics. It is frequent in North America and rare among Bedouin Arabians39, 41. It is considered a typical disease of childhood and adolescence, but it may affect patients in all age ranges39, 41. The studies estimate that 20% of the population in general will have RAS before adult age38. Lesions are less recurrent and less severe as a result of aging31, 38. Children from privileged social backgrounds are more commonly affected than lower income children 31, 45. Some population groups, especially medical, dental medicine students, or those who are high-achievers have 50% increased prevalence of RAS38, 39. Some authors refer to the occurrence of RAS in the ratio of two women to one man. Lehner, however, believes that this difference is smaller and practically nonexistent in clinical practice39.
Etiopathogenesis
The definite causes of RAS lesions are still obscure19, 35. Some authors suggested that predisposing factors and associated conditions could attribute to the development of the affection14, 38, 45. Aphthous lesions induced by drugs have not been fully studied yet4.
1- Local trauma
Trauma has been reported by patients, clinicians and scholars as the triggering factor for the development of RAS lesions. According to patients, the most common causes of traumas are: tooth brushing, dental floss, chewing gum, fishbone, poor dental occlusion, injections and dental treatment27, 38, 39. Aphthous lesions occur in areas poorly keratinized of the oral mucosa and for that reason, they are very sensitive38, 39.
2- Smoking
The relation between smoking and reduced incidence and severity of RAS has been recognized for years2, 27. Some patients notice the growing of RAS after smoking cessation and remission once smoking is restarted22, 31, 38. Dorsey suggested that the mucosa hyperkeratosis caused by tobacco acts as local protection factor to RAS ulcers39. However, health care professionals should not encourage smoking since it predisposes to cardiovascular diseases, neoplasm and pulmonary diseases17.
3- Psychological status
In 1957, Sircus et al. reported that emotional stress precedes the development of initial RAS episodes in 60% of the cases and the onset of recurrent episodes in 21% of the cases27, 31, 38. However, there are few data that can confirm this correlation.18, 31, 38
4- Menstrual cycle
A minority of patients with RAS have cyclic oral ulceration in the luteinic phase of the menstrual cycle27. Doubly demonstrated an increase in the incidence of new ulcers seven days after ovulation probably because of increased levels of progesterone and reduced levels of estrogen. Bishop, Harris and Trafford reported an improvement in ulcers in 30 women of 33 studied subjects in which aphthae were induced in the luteinic phase and treated with estrogen therapy in sufficient doses to suppress ovulation39. However, a careful review of the literature conducted by McCartan and Sullivan did not find association between RAS and female sexual hormones31, 38. However, some patients do not present the disease after estrogen administration38, 39.
5 - Biological Agents
5.1 - Bacteria
An association between RAS and Streptococcus viridans was suggested as important in the pathogenesis of the disease. The bacteria act as antigens stimulating the immune reaction. However, studies indicated that Streptococcus viridans is not specific to RAS31. According to Scully et al. Helicobacter pylori was detected in lesion tissue and through PCR in 72% of the examined ulcers. However, the frequency of IgG antibodies for H. Pylori was not increased in these patients31.
5.2 - Virus
Many authors indicated some viral agents as the cause of RAS. Among them we can mention herpes simplex virus (HSV), Varicella zoster virus, Epstein-Barr virus, Cytomegalovirus and adenovirus37, 38, 50. HSV was suggested as the cause of RAS, but studies confirmed that it is not always found in RAS lesions and only in patients with RAS who are serum positive. The proposal of a correlation between Epstein-Barr virus and recent ulcerative lesions of RAS and Behcet's disease1 was not accepted because it was based on a small sample. Human herpes virus-6 (HHV-6) DNA is not detected in most lesions of RAS even though many patients have IgM antibodies for HHV-6. It is known that HHV-6 DNA and HHV-7 are really detected in peripheral blood monocytes of patients with RAS31. Antiviral agents, such as acyclovir, effective against HSV, do not seem to have beneficial effects on RAS51.
6- Genetic factors
Thirty years ago, Ship believed that RAS had a family basis.38 More than 40% of the patients with RAS have family history of oral ulceration and develop oral ulcers younger and have symptoms that are more severe than those in their parents' generations31. There is also a great incidence of RAS in identical twins21, 31, 38. There is no consistent association between RAS and specific serology for a determined HLA antigen or haplotype 31. According to Kuntz et al. in 1977, HLA-B12 antigen was present in patients with RAS in great proportion. HLA-B5 was not increased in these patients, even though it was present in 75% of the cases of Behcet's syndrome41. The authors noticed that the combination HLA-A2 with HLA-B12 and HLA-A29 with HLA-B12 were frequent in patients with RAS suggesting that RAS susceptibility can be related to haplotypes 41.
7 - Food Hypersensitivity
Some studies showed a significant number of atopics among patients with RAS31. Some patients correlated the onset of oral ulcers with intake of a certain type of food27, 31, 38. Studies of small groups of patients identified some foods as responsible for the onset of RAS. They are: gluten, benzoic acid, sorbic acid, cinamaldehyde and azo dyes38. In some rare cases, the diet improved with RAS31.
8- Gluten-Sensitive Enteropathy (GSE)
It was suggested in a study that 5% of the patients with RAS are prone to GSE. Patients rarely have gastrointestinal symptoms or other clinical characteristics of GSE. Normally, they had folate deficiency, some times reticulinic antibodies of IgA class and/or antigliadine antibodies31. Haplotype HLA-DRW10 and DQW1 can predispose patients with GSE to RAS. Some patients with RAS, even without clinical or histopathological evidence of GSE, can have a good response to a gluten-free diet31.
9 - Hematological deficiency
Many studies conducted in the US, England and Spain have demonstrated that iron, folic acid and B12 vitamin hematological deficiency is twice more common in patients with RAS than in those that do not have the pathology22, 31, 34. Nolan et al. referred that patients with vitamin deficiency improve significantly recurrent aphthae after therapeutic replacement of vitamins B1,B2 and B6.13
10 - Immunology
Study of peripheral blood of apparently healthy people with RAS showed abnormalities in the immune system: depression or inversion of CD4:CD8 (especially in people with severe RAS), increase in receptors gd+ of T cells and increase in production of a-tumoral necrosis factor production17, 22. Other studies suggested cellular immunity disorder involving T lymphocytes as a predisposing factor48. Although immunological damage of epithelial cells is the common route for the development of RAS lesions, the antigens responsible for starting the process remain undetermined38. Humoral immunodeficiency can cause aphthous-like ulceration, but in most of the patients with RAS, the level of serum immunoglobulins is normal.32,33
Associated systemic diseases
Oral mucosa lesions can be represented my manifestations of dermatological and systemic diseases, reactional lesions or occult neoplasm44. A successful treatment of RAS depends on precise diagnosis, classification of the disease and recognition of possible factors or associated diseases22, 31, 38. They are: acute vulva ulcer, Behcet's disease15,26,36,40, MAGIC syndrome (oral and genital ulcers with cartilage inflammation), FAPA syndrome (fever, aphthous, pharyngitis and adenitis)12, Sweet syndrome41, cyclic neutropenia, aphthous-like AIDS ulceration9, 33, hematic deficiency, gluten-sensitive enteropathy (celiac disease), intestinal inflammatory disease, and Reiter's syndrome.22,38,47
Lesions in AIDS patients tend to be as large as major and disabling lesions as in complex aphthosis. These RAS lesions normally affect subjects who have CD4 count below 50/mm3.13, 22, 23, 25, 38 Differential diagnosis in this state of profound immunodepression includes infectious or drug-induced oral ulcers. The diagnosis of aphthous-like ulcers associated to AIDS is an exclusion criteria38. Many studies showed that patients with RAS can have deficiencies of iron, zinc, folic acid, vitamins B1, B2, B6 and B12. Hematological assessment of these elements should be considered in all patients with complex aphthosis and with persistent symptoms of poor absorption and nutritional deficiency38. For many years, gastrointestinal diseases were associated to RAS. According to Dubois and Van den Berghe, the word "sprue", very important in gastrointestinal disease, derives from the Dutch word "spruw", which means aphthosis. Simple or complex aphthosis can precede, coexist or serve as a landmark in worsening of an intestinal inflammatory disease38.
Clinical Manifestations
RAS can affect various sites of the oral mucosa and they are clinically presented in three main forms: minor, major and herpetiform ulcer10, 39, 40, 41. Even though they are presented differently there are some common characteristics: shape, depth, edema and pain. Most of the times they are circular, shallow and painful11, 39, 41. All forms can recur within few days or months. Natural progression leads to spontaneous cure in most cases39.
Minor aphthous form is the most common variety, occurs in approximately 80% of the cases of RAS. They are circular, shallow and normally maximum 5 mm diameter in size. They can present a white-grayish pseudomembrane surrounded by a. erythematous halo. It affects labial and jugal mucosa, the floor of the mouth and rarely the gums, palate or tongue dorsum. The progression is normally to spontaneous cure in a period that varies from 10 to 14 days, without scars39, 41.
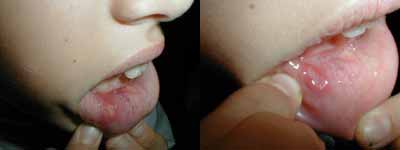
Figure 1. Minor aphthous lesion.
The major form is rarely present, but it has a very severe progression. It is known as Sutton's ulcers or periadenitis mucosa necrotica recurrens. They represent 10 to 15% of the cases of RAS. They normally appear after puberty, chronically and persist for 20 years or more. Lesions are characterized for being circular and sometimes oval. The size can exceed 1 to 3 cm in diameter. The ulcers occur more frequently in the lips and soft palate, but they can also be seen in any region of the oral mucosa. They are very painful and can persist for over 6 weeks, healing slowly and progressing to cure without the need therapeutic intervention. They can leave a scar5, 8, 11, 22, 39, 41.
The other variety of RAS is herpetiform ulcer. It is the less common form and it affects 5 to 10% of the patients. The lesions are characterized for being multiple, small and painful. In some cases, there can be up to 100 ulcers simultaneously. Small lesions are grouped and they can get together and become greater, with irregular borders22, 31, 38, 41. They affect any region of the oral cavity, and do not prefer any specific site. They are preferably seen in females and affect older patients when compared to other forms41. Owing to size and depth of lesion, they take 7 to 30 days to heal and can leave scars38. Possibly, HLA-B12 acts as a pathogen-specific receptor. There may also be antigen determinants of some exogenous pathogens that simulate HLA antigens9. The distribution of HLA antigen in patients with minor and major ulcers is similar, but the close association with HLA-B12 occurs in patients with herpetiform ulcers9. Another interesting classification of RAS is: simple aphthous x complex aphthous. The former represents lesions that cure within 1 to 2 weeks and recur again. Complex aphthosis is presented with a severe picture, deep, large, numerous and painful lesions. New lesions develop as the old ones are scaring and can be associated to genital or perianal lesions38.
Progression
The steps of natural evolution of RAS lesions were summed up by Stanley, who divided them into four stages: prondromic, pre-ulcerative, ulcerative and healing39. Stage I occurs in the first 24 hours before the onset of lesions and it is characterized by numbness, itching, pain and local hyperemia, without any clear clinical manifestation. Some patients do not report this stage39. Microscopically, mononuclear cells start to infiltrate the epithelium and there is edema38. Stage II or pre-ulcerative stage can last for 18 hours to 3 days. Painful sensation is moderate to very severe. The apthae starts as a hardened erythematous small macula or papule. They can be solitary or multiple, circular or oval, depending on the location, involved by a erythematous halo. When in the presence of jugal mucosa and lips, lesions are circular. If mouth and labial sulci are affected, the lesions are oval39. Stage III or ulcerative stage lasts for 1 to 16 days. Lesions are painful from start. Clinically, maculae and papule of stage II expand and ulcerate, put they reach the maximum size only between the 4th and 6th day after the onset of the clinical picture. Lesions are gradually recovered by a gray and yellow membrane, surrounded by a red halo. In 2 to 3 days, pain ceases abruptly, but there is still the residual discomfort. The fourth and last stage is called healing and can last for 4 to 35 days38, 39. Scars are common in the major aphthous form and it is related to depth of lesion and occurrence of local tissue necrosis39.
Treatment
Treatment of oral cavity lesions is still a dilemma for the medical teams responsible for managing them30. Lesion severity (number, location, size and duration) and its effects on the patients (degree of discomfort, effects during feeding, and quality of life) should be evaluated. If RAS is severe or frequent, the possibility of having lesions associated to asymptomatic systemic disease or other conditions should be considered3, 22.
There are various treatment options for RAS: observation, systemic disease treatment, topical and systemic drugs, transformation of aphthous ulcer into wound and palliative treatment22.
OBSERVATION OF LESION
When choosing a treatment regime it is important to know that RAS cures spontaneously and that the frequency of recurrence, duration of lesion and degree of discomfort are individualized. Oral examination should be carefully performed16. If ulcers are small, cause little pain and are not frequent we can choose to let it evolve spontaneously22.
TREATMENT OF ASSOCIATED SYSTEMIC DISEASE
Introduction of the appropriate treatment for ulcerative colitis, celiac disease, Crohn's disease, Behcet's disease, or replacement therapy of iron, vitamin B12 and folate, can result in improvement of RAS22, 24, 30. However, compliance with gluten-free diets or the use of vitamins by people without associated systemic diseases has not shown any benefits22.
TOPICAL THERAPY
Drug selection is based on various practical criteria, such as number and location of ulceration, severity (local, duration, pain) of lesions, their effects on the patients and their general state, and use of other drugs. Normally, topical medication is preferred because it has little side effects and less likelihood of drug interaction. If lesions are severe and cause substantial morbidity, systemic drugs or topical drugs combined with systemic drugs are advocated7, 22.
ˇ Topical Corticosteroid
It is still the main resource for treatment of RAS in most of the countries. The wide range of different topical corticoids can reduce symptoms31. One orobase compound available is triamcinolone acetonide 14, 47. The safest compound is the combination orobase with strong corticosteroid such as fluonamide, clobetasole or halbetasole 22. Another effective alternative is dexamethasone elixir. This liquid corticosteroid is useful when there are multiple ulcers or when they are located in the soft palate or oropharynx. The elixir is used for gargling or mouth washing22. Aerosol spray of beclomethasone diproprionate is another type of corticoid and it can be useful in ulcers that are difficult to reach, such as those in the soft palate and tonsil pillars14, 22. A quick response to treatment has been shown in HIV patients with severe RAS treated with intralesional injection of triamcinolone acetonide 22. This technique can be used together with systemic therapy with corticosteroid, especially in cases of refractory major RAS. For smaller or herpetiform lesions, they can be considered an unnecessary invasion. 22
ˇ Topical Antimicrobial Agents
The use of clorexidine at 0.2% for mouth washing or gel at 1% can reduce duration of ulcer and increase the number of days without lesion31. However, it may cause dental spots or bitter breath31. Mouth washing with tetracycline can reduce pain, but causes dysgeusia, oral candidiasis and burning sensation in the pharynx14, 22, 31. In addition, it may not be used in children because it causes dental damage31.
ˇ Topical Analgesics
The use of benzidamine chloridrate does not benefit ulcers' cure. However, its use or the use of lidocaine gel in some cases can bring temporary relief of pain7, 31.
ˇ Other Topical Agents
Tooth pastes that stimulate the peroxidase enzyme of the salivary system are not effective. Sodium chromoglycate drops can provide mild relief of symptoms. Oral antiseptic sodium carbenoxolone can reduce severity of RAS31. Some topical immunomodulating agents are mentioned as beneficial for the treatment of RAS, such as: azelastine, human interferon a-2 in lotion, topical cyclosporin, topical amino salicylic acid-5 and gel of Prostaglandin E2 31. Sucralfate and amlexanox can reduce duration of symptoms and increase time of remission of RAS22, 31, 39. Cimetidine can also be therapeutically successful31.
SYSTEMIC THERAPY
Systemic immunosuppression is sometimes necessary considering the limited efficacy of topical agents, when severe pain and/or duration of ulcers is long31. The most common agent is corticoid. Other drugs that are used are: cyclosporin, colchicine, pentoxiphylline, dapsone and thalidomide. The use of these agents in the prondromic stage can abort the formation of ulcers22, 24.
ˇ Corticosteroids
Prednisone is an effective corticoid that has been used successfully for many years in the treatment of RAS, both isolated and in combination with topical corticoids and analgesics. it has shown efficacy and safety in children and adults with AIDS or positive HIV who have major disabling RAS or non-specific aphthous-like ulcers. It can be used for a short period of time, 4 to 5 days with 60-80mg/day dose, or for more than a week with gradual reduction. This regimen can be associated to topical corticoid, intralesional injection of triamcinolone acetonide and analgesics (such as lidocaine gel, NSAIDs or narcotics). If oral candidiasis is developed, it should be treated with the appropriate antifungal agents20, 22.
ˇ Levamisole
Levamisole can be used because it promotes chemotaxia of leukocytes and increases in phagocytic activity of neutrophils. In small doses, it can maximize the immune response, reestablishing reactivity of anergic patients; in high doses, it can act as immunosuppressant 30. In 1980, Miller reported the use of levamizole in RAS to reduce the incidence and number of ulcers. In some occasions, there is significant reduction of duration and size of lesions30. It can be administered for 2 weeks in a dosage of 150mg/day, divided into 3 doses for 3 days, followed by a period of 11 days without the drug. This regimen can be continuously repeated by patients with frequent exacerbation. The episodes become infrequent. If there is insomnia, nausea, odynophagia or dizziness, hyperosmia, dysgeusia and agranulocytosis, the dose can be reduced to 100 or 50mg/day5.
ˇ Colchicine
Colchicine has clinical efficacy in some patients with RAS and can be used in 1.5mg/day for 2 months, causing reduction of pain and frequency of ulcers. Patients can present abdominal pain or diarrhea. If used for prolonged periods of time, it induces fertility in young patients. In some cases, the combination of colchicine and thalidomide brought benefits to RAS30, 31, 47.
ˇ Pentoxiphylline
Results of open studies showed that the use of pentoxiphylline (400mg 3x/day, for one month) can lead to reduction of number of RAS episodes for over 9 months. However, the results of another study did not confirm that pentoxiphylline reduce the recurrence of RAS after interruption of therapy. This drug blocks the adherence of neutrophils and specific antigens for the activation of T lymphocytes20, 30.
ˇ Dapsone
According to Popovsky and Camisa, dapsone was useful to suppress RAS. This experience was based on a study by Ghate and Jorrizo.30 Affected patients should dose glucose-6-phosphate dexydrogenase before treatment. Subjects who present deficiency of the enzyme can not use dapsone because of the risk of severe hemolytic anemia. Doses of 100 to 200mg/day are necessary to induce remission. It is recommendable to associated dapsone to antioxidant vitamin E, 800IU/day to reduce the level of hemolysis 30.
ˇ Thalidomide
Thalidomide is a hypnotic sedative introduced in 1950 and withdrawn from the market owing to its teratogenicity. It has currently been reintroduced in selective cases to manage inflammatory or immune-mediated diseases. It has been suggested that it promotes the inhibition of a-tumor necrosis factor production. It is still the most effective agent to treat RAS. It promoted remission in 50% of the patients treated in a randomized trial22, 31. Open and double-blind studies showed efficacy also in HIV positive patients with oral lesions6, 31, 49. Serious considerations should be made about the side effects of thalidomide, such as sedation, neuropathy, reduction of libido, cutaneous rash, neutropenia, constipation and teratogenicity 6, 20, 22, 31. The thalidomide dose suggested for the treatment of severe RAS is 100mg/day14, 30, 46.
ˇ Other agents
Low doses of IFN-alpha 1200 UI/day for mouth washing can induce remission of minor RAS lesions. HIV patients with gingivitis and severe RAS can benefit from the use of oral IFN-alpha, 150UI/day, isolated or combined with AZT30. Transference factor and gammaglobulin therapy were suggested as effective, but detailed studies are still required to confirm these preliminary observations31.
CONVERSION OF RAS INTO WOUNDIt has been proposed that by transforming the aphthous ulcer into wound, we can start spontaneous cure. The methods to make the conversion are: biopsy, chemical induction (cauterization with silver nitrate) or chemical induction (laser ablation)14, 22, 31. Surgical removal, debridment, or laser ablation of ulcers are not practical techniques and do not provide great advantages. 31
ConclusionRAS is a very common oral mucosa disorder, common in cities and presenting recurrent character. Its etiology is multifactorial and encompasses local and systemic causes, deserving careful investigation that involves various medical specialties. Despite the great clinical, immunological, hematological and microbiological trials, etiology remains obscure. Currently, some studies make believe that RAS is associated to immune response of the oral mucosa. Recurrent affection affects the routine of the patient, sometimes leading to disability to labor activities. It deserves attention from health care professionals involved in treatment and control of the disease. Therapy is not specific, and it is important to treat systemic disease when they are the cause of RAS. Various drugs have been used to control lesions and they can be used topically or systemically, despite their limited efficacy in some cases.
References1. Amador VAR, Pedraza LE, Topete RO. Frequency of oral conditions in a dermatology clinic. International journal of Dermatology 2000;39:501-05.
2. Axell C & Henricsson V. Association between recurrent aphthous ulcers and tobacco habits. Journal of Dental Research 1985;93:239-42.
3. Baughman RA. Recurrent aphthous stomatitis vs. recurrent herpes: do you know the difference? J Ala Dent Assoc 1996;80(1):26-32.
4. Boulinguez S, Cornee-Leplat I, Bouyssou-Gauthier ML, Bedane C, Bonnetblanc JM. Analysis of the literature about drug-induced aphthous ulcers. Ann Dermatol Venereol 2000;127(2):155-58.
5. Burruano F, Tortorici S. Stomatite aftosa major (malattia di Sutton). Etiopatogenesi, quadri istologici ed aspetti clinici. Minerva Stomatol 2000;49(1-2):41-50.
6. Calabrese L, Fleischer AB. Thalidomide: current and potential clinical applications. Am J Med 2000;108(6):487-95.
7. Carpenter WM, Silverman S. Over-the-counter products for oral ulcerations. J Calif Dent Assoc 1998 Mar;26(3):199-201.
8. Chung JY, Ramos-Caro FA, Ford MJ, Mullins D. Recurrent scarring ulcers of the oral mucosa. Sutton disease (periadenitis mucosa necrotica recurrens). Arch. Dermatol. 1997 Sep;133(9):1162-3, 1165-6.
9. Claessen FA, Vos MJ, Simoons-Smit AM, Debets-Ossenkopp YJ, Perenboom RM. Manifestaties van histoplasmose. Ned Tijdschr Geneeskd 2000;144(25):1201-5.
10. Eversole LR. Diseases of the oral mucous membranes In: Millard D & Mason DK (eds.) World Workshop on Oral Medicine. Chicago: Year Publishes; 1989. p.54-121.
11. Eversole LR. Immunopathogenesis of oral lichen planus and recurrent aphthous stomatitis. Seminars in Cutaneous Medicine and Surgery 1997;16(4):284 -294.
12. Feder HM. Periodic fever, aphthous stomatitis, adenitis: a clinical review of a new syndrome. Curr Opin Pediatr 2000;12(3):253-6.
13. Ficarra G. Oral ulcers in HIV-infected patients: an update on epidemiology and diagnosis. Oral Disease 1997 may;3 suppl 1:s183-9.
14. Filho ACNN, Bettega SG, Lunedo S, Gortz F, Maestri JE, Abicalaffe MD. Estomatite Aftóide Recidivante: Revisăo e Proposta de Protocolo no seu Atendimento. Arquivos da Fundaçăo Otorrinolaringologia 1999;3(4):172-6.
15. Frances C. Dermato-mucosal manifestations of Behcet´s disease. Ann Med Interne 1999 nov;150(7):535-41.
16. Greenspan D, Greenspan JS. HIV-related oral disease. Lancet 1996;348(9029):729-33.
17. Guranska N, Urbaniak B, Lewkowicz P, Tchorzewski H. Recurrent aphthous ulcers: the etiology with special reference to immunological theories. Pol Merkuriusz Lek 2000;8(44):113-17.
18. Heft M & Wray D. Anxiety levels in recurrent aphthous stomatitis (RAS) patients. Journal of Dental Research 1982;39:212-18.
19. Hornstein OP. Aphthae and aphthous lesions of the mouth mucosa. HNO 1998;46(2):102-11.
20. Lacosta Nicolas JL, Martinez Iniguez JC. Treatment of recurrent aphthous stomatitis: A bibliographic review. Rev Clin Esp 1998;198(4):234-36.
21. Lehner T. Progress report: oral ulceration and Behcet's syndrome. Gut 1977;18:491-511.
22. MacPhail, L. Topical and Systemic Therapy for Recurrent Aphthous Stomatitis. Seminars in Cutaneous Medicine and Surgery 1997;16(4):301-7.
23. Macphail LA, Greespan JS. Oral ulceration in HIV infection: investigation and pathogenesis. Oral Disease 1997 may;3(suppl 1):s190-3.
24. Mcbride DR. Management of aphthous ulcers. Am Physician 2000;62(1):149-54, 160.
25. Mccullough MJ, Firth NA, Reade PC. Human immunodeficiency virus infection: a review of mode of infection, pathogenesis, disease course, and the general and clinical manifestations. Aust Dent J 1997 feb;42(1):30-7.
26. Medina LM, Rubio JLC, Garcia ET, Centeno NO. Utility of HLA Typing in the Differential Diagnosis of Severe Aphthosis and Behcet's disease. Dermatology 2000;280-1.
27. Miziara ID. Estomatite Aftóide Recidivante. Revista Brasileira de Otorrinolaringologia 1995;61(5):418.
28. Murray LN, Amedee RG. Recurrent aphthous stomatitis. J La State Med Soc 2000 jan;152(1):10-4.
29. Petersen MJ, Baughman RA. Recurrent aphthous stomatitis: primary care management. Nurse Pract 1996 may;21(5):36-40, 42, 47.
30. Popovski J, Camisa C. New and emerging therapies for diseases of the oral cavity. Dermatologic clinics 2000;18(1):113-25.
31. Porter SR, Hegarty A, Kaliakatsouu F, HodgsonTA, Scully C. Recurrent Aphthous Stomatitis. Clinics in Dermatology 2000;18:569-578.
32. Porter SR & Scully C. Aphthous stomatitis - an overview of aetiopathogenesis and management. Clinical and Experimental Dermatology 1991;16:235-43.
33. Porter SR & Scully C. Primary immunodeficiency. In: Jones JH & Mason DK (eds.) Oral Manifestations of Systemic Disease. London: Balliere Tindall; 1990. p.112-62.
34. Porter SR, Scully C, Flint SR. Haematological status in recurrent aphthous stomatitis compared with other oral disease. Oral Surgery, Oral Medicine, Oral Pathology 1988;66:41-4.
35. Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit. Rev. Oral Bil. Med 1998;9(3):306-321.
36. Rees T D, Binnie WH. Recurrent aphthous stomatitis. Dermatol Clin 1996 apr;14(2):243-56.
37. Reichart PA. Oral ulcerations in HIV infection. Oral Disease 1997 may;3(1 suppl):s180-2.
38. Rogers III RS. Recurrent Aphthous Stomatitis: Clinical Characteristics and Associated Systemic Disorders. Seminars in Cutaneous Medicine and Surgery 1997;16(4):278-83.
39. Rogers III RS. Recurrent Aphthous Stomatitis: Clinical Characteristics and Evidence for an Immunopathogenesis. The Journal of Investigative Dermatology 1977;69:499-509.
40. Rogers III RS. Recurrent aphthous stomatitis in the diagnosis of Behcet´s disease. Yonsei Med J 1997 dec;38(6):370-379.
41. Scully C, Porter SR. Aphthous Stomatitis: an overview of aetiopathogenesis and management. Clinical and Experimental Dermatology 1991;16:235-43.
42. Scully C, Yap P, Boyle P. IgE and IgD concentrations in patients with recurrent aphthous stomatitis. Arch Dermatol 1983;(119):31-4.
43. Ship JA. Recurrent aphthous stomatitis. An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996 feb;81(2):141-47.
44. Siegel MA. Strategies for management of commonly encountered oral mucosal disorders. J Calif Dent Assoc 1999;27(3):210-12, 215,218-19 passim.
45. Silva MR, Fernandes NC. Afecçőes das Mucosas e Semimucosas. JBM 2001;80(3):50-66.
46. Torras H, Lecha M, Mascaro JM. Thalidomide in the Treatment of Recurrent, Necrotic, and Giant Mucocutaneous Aphthae and Aphthosis. Arch Dermatol 1979;115:636-37.
47. Tüzün B, Tüzün Y, Wolf R. Oral Disorders: Unapproved Treatments or Indications. Clinics in Dermatology 2000;18:197-200.
48. Ueta E, Umazume M, Yamamoto T, Osaki T. Leukocyte dysfunction in oral mucous membrane diseases. J Oral Pathol Med 1993;(22):120-5.
49. Weidle PJ. Thalidomide for aphthous ulcers in patients infected with the human immunodeficiency virus. Am J Health Syst Pharm 1996 feb;53(4):368, 371-2, 378.
50. Woo SB, Sonis ST. Recurrent aphthous ulcers: a review of diagnosis and treatment. J Am Dent Assoc 1996 aug;127(8):1202-13.
51. Wormser GP, Mack L, Lenox T et. al. Lack of effect of oral acyclovir on prevention of aphthous stomatitis. Otolaryngology and Head and Neck Surgery 1988;98:14-7.
[1] Coordinator, Advanced Learning in Otorhinolaryngology, Clinic Professor José Kós. Member of the staff of Clinic Professor José Kós.
[2] Resident physician, Advanced Learning, Clinic Professor José Kós.
[3] Resident Physician, Advanced Learning, Clinic Professor José Kós.
Address correspondence to: Dra. Paula Moreno Fraiha - Rua: Padre Elias Gorayeb, 15/ 606
Tijuca - Rio de Janeiro - RJ - 20520-140


