INTRODUCTIONElective neck dissections are considered staging procedures for squamous cell carcinomas of he upper aerodigestive tract, indicated when there is greater than 20% likelihood of regional occult metastases1. Selective dissection, with less morbidity and minimum sequela, has two purposes, although dissections are normally performed with a therapeutic purpose. The justification for overtreating approximately 70-80% of the cases is attributed to worse results observed when neck dissection is performed after recurrence2, 3. The superiority of elective dissection over required dissection has not been confirmed, though1. An observation approach only, however, is not recommended if the patient can not be closely followed up. It hinders early diagnosis of recurrence and reduces quickness in surgical salvage procedures, since in some cases, metastases have explosive growth after resection of the primary tumor. Selective dissections, whose extension is based on lymphatic tumor spread studies in different anatomical sites, proved to be appropriate to treat neck at N0 stage. If there is metastatic disease, the occasional regional recurrence will probably be located in the areas included in the dissection4, 5.
The present study intended to analyze occult neck lymphatic metastatic spread, assessing its location and risk factors in patients with squamous cell carcinomas submitted to elective neck dissection.
MATERIAL AND METHODThe study consisted of retrospective analysis of medical charts of 428 patients with squamous cell carcinoma of upper aerodigestive tract, with no previous treatment, submitted to elective neck dissection at the Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, between 1977 and 1996.
Patients were grouped according to the four anatomical regions that hosted the tumors: mouth, oropharynx, larynx and hypopharynx. There were 5 levels of lymphatic chain (submandibular I, high carotid jugular II, middle carotid jugular III, low carotid jugular IV, and spinal V). The identification of the levels was made by the surgeon immediately after removal of the surgical piece. Neck dissection were classified according to the criteria proposed by the American Head and Neck Society6. The anatomic pathological analysis employed, as a routine, 2 sections for each lymph nodes. We analyzed the correlation between primary tumor site, T staging (TNM classification by UICC-AJCC/1997), sex, age, differentiation of histologic grade and presence of metastases. We assessed the presence of metastases in different lymphatic chains compared to tumors in different anatomic regions.
The impairment of different lymphatic levels considered the frequency of metastases in each chain and the total number of patients whose dissection included the respective level.
Statistical analysis used chi-square test to evaluate the differences between categorical quantitative and qualitative variables.
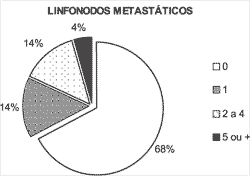
Figure 1. Number of metastatic lymph nodes.
Among the patients, 390 were male and 38 were female subjects. The mean age of patients was 57 years, ranging from 26 to 85 years. Larynx was the primary tumor site in 180 cases, the mouth in 175, the oropharynx in 38 and the hypopharynx in 35. As to staging, we found 15 cases of T1, 121 cases of T2, 201 cases of T3 and 83 cases of T4, in addition to 8 cases whose information was not enough to conclude about TNM restaging (1997). Three hundred and thirty-six unilateral radical dissections were performed, as well as 43 bilateral radical procedures, 22 unilateral selective ones and 6 mixed bilateral procedures (radical + selective). On average, 35 lymph nodes were analyzed per patient.
The rate of occult metastasis was 32% in mouth tumors, 31% in oropharynx, 40% in hypopharynx and 26% in the larynx. In laryngeal tumors, there was proportional ratio between staging of primary tumor and occult metastasis transformation, not observed in the other sites (Table 1).
Metastases affected one lymph node in 14% of the patients and multiple nodes in 18% (Figure 1).
There was lower incidence of occult metastases in females. Age, sex and grade of histologic differentiation did not show significant correlation with index of histology metastases (Table 2).
In mouth, oropharynx and larynx tumors, level II was the most affected one. In tumors of hypopharynx, there was predominance of level III. High spinal lymph nodes (level V) presented metastases in 10 patients (2.3%) (Table 3).
Neck dissection was performed bilaterally in 70 patients, in which only 7 (10%) presented bilateral metastases (pN2c).
Table 1. Occult metastasis in each T stage and anatomical site.

P = standard deviation
n.s. = not significant
Table 2. Clinical and histologic factors related to presence of occult metastases.
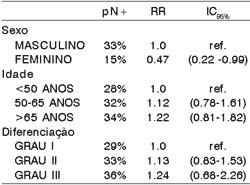
pN+ = positive histologic lymph node
RR = relative risk
IC = confidence interval
Ref = reference
Table 3. Level of occult metastases in different anatomical sites.

Distribution of metastases in squamous cell carcinoma of upper aerodigestive tract presents a predictable pattern, with small individual variations. The results of the present study are similar to those observed by other authors, with minor changes2, 3, 4, 5, 7. Lymph node levels impaired with occult metastases are the same observed in advanced stages of the disease8. Dissection of level I cases is only justified in mouth lesions. Our sample showed that mouth lesions (regardless of the mouth site) presented higher metastases generation in level I when lesions were on the floor of the mouth and lower gum, whereas tongue lesions had the predominance level II involvement. In pharynx and larynx only levels II and III are impaired in a significant portion of the patients. High spinal lymph nodes (V) were impaired in a small number of patients, since this level is normally involved in rhinopharynx neoplasm cases9, and its exclusion from dissection can reduce the morbidity caused by manipulation of 11th cranial nerve (spinal nerve). As to dissection in level IV, it can be considered an opportunity procedure. It occurs during supraomohyoid dissection (levels I, II and III), indicated in mouth lesions, despite the fact that levels III and IV can be the first drainage stations in lesions of the tip of the tongue and posterior region of the mouth floor (skip metastases)10,11. As to bilateral metastases, they were found in only 10% of the patients submitted to bilateral dissection, results that did not confirm the conduction of bilateral elective dissection when there is no metastasis on the predominant side. In addition to midline lesions and cross anatomic lymphatic drainage sites, indication of bilateral neck dissection should also consider the rates of contralateral regional recurrence, not analyzed by the present study12, 13.
Metastases occurred predominantly in the normally reactive lymph nodes chains. This fact can reflect the stimulus of the closest drainage chain, but the reactive lymph node can also provide more appropriate conditions for tumor growth. The presence of metastases at lower levels, normally less reactive, is associated to poor prognosis9, 10.
The small number of female patients did not allow conclusions about the causes of a lower incidence of occult metastases in this group, since lymphatic metastases normally occur similarly in both genders. It was observed a higher rate of occult lymphatic metastases among elderly patients and in those with less differentiated tumors, although these differences were not statistically significant (table 2). There is a report of greater incidence of metastases in black patients but in the present study we did not analyze racial characteristics owing to the great miscegenation of the Brazilian population13.
The rate of occult metastases in mouth tumors was similar in primary lesions of different sizes (Table 1). The proportion of patients clinically with metastases is evidently higher in more advanced stages, but mouth tumors, differently from laryngeal tumors, present high level of metastases, even in initial stages. A similar behavior was observed in oropharynx and hypopharynx tumors. These results suggest that patients with extensive mouth lesions, oro and hypopharynx lesions can benefit from non-invasive diagnostic methods that investigate the presence of occult neck lymphatic metastases. Since the presence study used conventional histopathologic analysis, a significant number of micrometastases was probably not identified, but the clinical meaning of micrometastases in squamous cell carcinomas of the head and neck have not been determined yet14, 15, 16, 17. Generically speaking, we can say that selected neck dissections are appropriate procedures for staging and they provide removal of most subclinical metastases in patients with upper aerodigestive tract squamous cell carcinoma18, 19.
CONCLUSIONSElective neck dissection allow identification of the presence of occult metastases in a high number of patients, especially at lymphatic level I for oral cavity and levels II and III for pharynx and larynx. As to risk factors, age, sex and grade of histology differentiation, they were not significant determinants for the presence of occult neck metastases.
REFERENCES1. Goepfert H. Evolution of elective neck dissection. Proceedings of 5th Conference on Head and Neck Cancer, San Francisco; 2000:1-7.
2. Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg 1988;10(3):160-167.
3. Shah JP. Cervical lymph node metastases. Diagnostic, Therapeutic, and prognostic implications. Oncology 1990;4(10):61-69.
4. Byers RM. Modified neck dissection. A study of 967 cases from 1970 to 1980. Am J Surg 1985;150(4):414-421.
5. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg 1990;160(4):405-409.
6. Robbins KT, Denys D. The American Head and Neck Society's revised classification for neck dissection. Proceedings of 5th Conference on Head and Neck Cancer, San Francisco; 2000:365-371.
7. Davidson BJ, Kulkarny V, Delacure MD. Posterior triangle metastases of squamous cell carcinoma of the upper aerodigestive tract. Am J Surg 1993;166(4):395-398.
8. Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29(6):1446-1449.
9. Klimek T, Glanz H, Dreyer T. Histomorphological characteristics of non-metastatic lymph nodes in patients with head and neck cancer according to their site in the neck. Acta Otolaryngol 1996;116:336-340.
10. Byers RM, Weber RS, Andrews T. Frequency and therapeutic implications of skip metastases in the neck from squamous carcinoma of the oral tongue. Head Neck 1997;19:14-19.
11. Traserra J. Adenopatias Cervicales Metastasicas. Sociedad Española de Otorrinolaringologia y Patologia Cervico-Facial. XIII Reunión Anual Madrid; 1980. p. 40-43.
12. Feind CR, Cole RM. Contralateral spread of head and neck cancer. Am J Surg 1969;118(5):660-665.
13. Kowalski LP, Medina JE. Nodal metastases: Predictive factors. Otolaryngol Clin North Am 1998;31(4):621-637.
14. Gillies EM, Luna MA. Histologic evaluation of neck dissection specimens. Otolaryngol Clin North Am 1998;31(5):759-771.
15. Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 1999;91:1113-1124.
16. Van Den Breckel MWM, Van Der Waal I, Meijer CJML. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissection. Laryngoscope 1996;106:987-991.
17. Wilkinson EJ, Hause L. Probability in lymph node sectioning. Cancer 1974;33(5):1269-1274.
18. Spiro JD, Spiro RH, Shah JP. Critical assessment of supraomohyoid neck dissection. Am J Surg 1988;156:286-289.
19. Spiro RH, Gallo O, Shah JP. Selective jugular node dissection in patients with squamous carcinoma of the larynx or pharynx. Am J Surg 1993;166(4):399-402.
[1] Surgeon, Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis - HOSPHEL, São Paulo, Brazil.
Study conducted at the Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis - HOSPHEL, São Paulo, Brazil.
Address correspondence to: Prof. Dr. Abrão Rapoport - Praça Amadeu Amaral, 47 cj. 82
Paraíso - São Paulo, Brazil -01327-010
E-mail: cpgcp.hosphel@attglobal.net


