IntroductionVocal fold polyp is a benign, hyperplastic well-defined lesion, normally located on the two anterior thirds of the vocal folds. It can be sessile or pediculated and pale or reddish in color. It affects all age ranges and does not have gender predominance, but it may be associated to smoking, pollution and vocal abuse1.
Various methods of surgical treatment have been proposed after failure of drug and voice therapy approaches. The purpose of the present study was to show our clinical and surgical experience with polyp lesions, correlating it to the international literature.
Material and methodWe conducted a retrospective study including all patients of the Ambulatory of Larynx, Discipline of Otorhinolaryngology, Medical School of ABC, between February 2000 and July 2001. We analyzed the following data: age, sex, clinical symptoms, personal history, habits (smoking, alcohol abuse), results of direct laryngoscopy and stroboscopy (if performed), diagnosis, treatment, pathology analysis and post-surgical follow-up (seven, 15, 30 and 60 days). Data were analyzed and the results were compared to those in the literature.
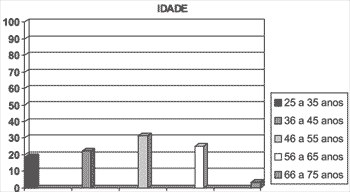
Graph 1. Percentage of patients with vocal polyps concerning age range.
Our sample consisted of 32 patients with diagnosis of vocal fold polyps, being 16 (50%) females and 16 (50%) males. Ages ranged from 25 to 75 years, mean age of 46.9 years (SD = 11.6).
The main clinical symptoms and personal history elements are listed in Tables 1 and 2.
Dysphonia was the most frequent symptom (42%), followed by globus pharyngeus (22%).
Smoking was reported in 56% of the patients and vocal abuse in 15%.
Out of 26 patients, 15 (57.5%) presented pediculated polyps and 11 (42%) had sessile polyps.
The patients with pale lesions presented longer progression of the disease (average of 2 years) as opposed to red polyps (6 months).
Left vocal fold was more frequently affected (56.25%; n = 18).
Twenty-six patients with polyps (81.25%) were treated with laryngeal microsurgery: grasping of polyp and placement to the midline, to visualize its insertion. Through the incision, more easily seen in sessile polyps, the lesion was completely removed with scissors. In sessile polyps, the lesion was carefully separated from the vocal fold epithelium and then the basis was resected.
In all cases, we planned postoperative voice therapy and all patients used analgesics after the surgery, if necessary. No preoperative therapy was conducted.
In four patients the lesions disappeared spontaneously without treatment and two patients gave up treatment.
Histopathologic findings in most polyps (95%) were fibrin exsudate interconnected by connective tissue or endothelial cells and neovascularization. In addition, atrophy and acanthosis, diffuse submucous edema, submucous infiltration with lymphocytes, fibroblasts and blood pigments were also reported. In red polyps, there was predominance of endothelial cells and neovascularization.
In 90% (n=29) of the patients there was edema and inflammatory signs in the larynx one week after the surgery and persistent dysphonia. After 15, 30 and 60 days, the percentage reduced to 60% (n=19), 30% (n=9) and 5% (n=1), respectively.
Table 1. Main clinical symptoms presented by patients with vocal polyps (N = 32 patients).CLINICAL SYMPTOMS / Nº of patients (%)(N = 32)
Dysphonia - 41.9 %
Globus pharyngeus - 21.9 %
Dyspnea - 21.9 %
Cough - 18.8 %
Throat clearing - 6.3 %
Dysphagia - 6.3 %
Epigastralgia - 6.3 %
Pain - 0 %
Table 2. Main habits and personal history of patients with vocal polyps (N = 32).HABITS AND PERSONAL HISTORY / Nº patients (%) (N = 32)
Smoking - 56.2 %
Alcohol use - 15.6 %
Vocal abuse - 25.1 %
Previous IOT - 6.3 %
GERD - 3.1 %
DiscussionVocal fold benign lesions are a significant problem for otorhinolaryngologists, because they are very common pathologies that require multidisciplinary treatment. When these lesions do not respond to drug and/or voice therapy approaches, the treatment option is surgical. The purpose of the surgery is to increase phonation function or to define the pathology diagnosis through biopsy2.
In our 18-month retrospective analysis, there was predominance of polyp lesions, a fact that shows growing incidence of this lesion, probably as a result of the dynamic changes in lifestyle or unfavorable environmental conditions, such as smoking and pollution1. Conversely, a greater number of patients have searched for medical services and the number of diagnoses has increased proportionally.
The pathogenesis is related to submucous vasodilatation of the vocal fold, resulting in increased permeability of the vascular wall with edema, predominantly on the anterior or middle third of the vocal fold, where the mechanical forces of vibration are more marked. This exsudate rich in protein can get organized and fibrotic, or start suffering hyaline or basophil degeneration. In cases of angiomatous polyps, there may be small and focal subepithelial hemorrhages, leading to the characteristic aspect (red color). All these mechanisms can be triggered by multiple factors, being vocal abuse the most expressive one (25%)1.
Histologically, there are different grades of epithelial hyperplasia and atrophy. Dikkers et al.3 reported that ultrastructural exam of benign vocal folds lesions do not provide additional information to conventional microscopy, whose diagnosis is very precise.
Characteristic combinations of histology findings contribute to the differentiation of clinical entities, such as polyps, Reinke's edema and nodules4, 5.
Most of our histology findings presented a combination of recent bleeding signs, iron and fibrin deposits and thrombosis, confirming the clinical diagnosis of polyps. It is expected to have a relation between histopathological presentation and lesion pathophysiology. It might be that vocal abuse induces greater vessel traumas, leading to hemorrhage, fibrin exsudate, thrombosis and capillary proliferation. Normally, the formation of polyps is an acute event, whose initial damage is perpetuated by recurrent movements of the lesion during phonation, inducing to recurrent capillary traumas.
At stroboscopy, Hirano et al.6 reported the main findings in patients with polyps. Similarly to our cases, the glottis presented incomplete closure during vibration, with irregularities of successive vibrations, reducing the amplitude of the affected vocal fold and interfering in the vibration movement of the non-affected vocal fold. The mucous wave was reduced in the affected side and in some cases, it was even absent.
In 1960, laryngeal microsurgery with suspension laryngoscope under general anesthesia was introduced. This modality of surgery provides excellent stability of surgical field, without the occurrence of reflexes in the patient, and certainly it provides good precision to identify the lesions. However, the intubation cannula can obstruct the surgical field and the control of phonation function can not be assessed intra-operatively. In phonosurgery, ideally, the surgeon should assess the patient's voice, and observe the vocal fold mucosa vibration during phonation.
To overcome such disadvantages, various methods have been developed. Laryngeal microsurgery with mirror under topic anesthesia, stroboscopic microsurgery of the larynx with mirror or rigid telescope under local anesthesia, and laryngeal microsurgery with suspension laryngoscope under neuroleptic anesthesia have been tried as alternative methods. However, these methods have not been frequently advocated because of difficulty in manipulation and scarcity of images in the surgical fields7.
Kojima et al.7,8 has used since 1991 laryngeal microsurgery with optic fiber and stroboscope under local anesthesia in an outpatient unit. Thanks to this technique, the surgeon uses both hands freely and performs voice and larynx monitoring, while an assistant inserts the endoscopic fiber through the nasal cavity and the patient pulls the tongue. In the results obtained with the use of the technique, there was improvement of postoperative vocal functions, increase maximum phonation times, with no intra-operative complications such as bleeding or aspiration. There was precise identification of the lesions and the larynx maintained a physiological position during the surgical act because there was no intubation cannula. The authors concluded that this type of surgery is well applied to remove polyps, cysts, nodules, not very extensive Reinke's edema and granulomas, in addition to being useful in biopsies. Moreover, it can be employed in patients with anatomical problems such as narrow mandible, neck immobility and other defects that prevented the performance of laryngeal microsurgery with suspension laryngoscope7. However, this technique requires the appropriate instruments, skills and quickness of the surgeon in such procedures, besides much collaboration of the patient, since topic anesthesia is used.
In 1972, Strong and Jako9 reported the first experience with the clinical use of CO2 laser to treat benign and malignant laryngeal lesions. Recently, CO2 laser has been carefully used owing to its thermal harmful potential to the mucous and muscular tissues of the vocal fold, leading to vocal deterioration. However, instrument refining during the yeas has transformed laser surgery into a precise procedure. In addition, other types of laser have been developed, such as the KTP/532 (potassium-titanium phosphate) and Nd:YAG laser.
In the retrospective study by Shapshay10, we observed greater advantages of laser over the cold techniques considering micro-precision and capacity of photo-coagulating small vessels on the mucous surface, a particular important fact in the case of angiomatous polyps. In the case of sessile polyps, laser is useful in the incision and dissection of the mucosa. Moreover, laser is indicated to treat vascular polyps and granulomas. Instrument improvement can lead to excellent results when used in small and pediculated lesions, such as nodules10, 11.
In our sample, all patients were submitted to laryngeal microsurgery with suspension laryngoscope at 95% (n=31) for considerable improvement of vocal quality and remission of other associated symptoms within 60 days after the surgery. Our results were excellent with the conventional technique and since we are a university hospital with resident training, surgery with topic anesthesia would have limited the procedure. Moreover, we do not have laser in our hospitals, discarding this procedure in our case.
Regardless of the technique used, all studied authors are unanimous in saying that voice therapy and appropriate postoperative follow-up (vocal hygiene) are mandatory elements for the success of treatment.
Woo et al.12 conducted a retrospective study addressing the main laryngovideostroboscopic findings in patients with persistent postoperative dysphonia (microsurgery or biopsy, with CO2 laser or not). As expected, etiology was widely variable. in most cases, they found vocal folds with reduced mobility owing to scaring, and also residual lesions after surgery, a fact that emphasizes the importance of documenting the voice and vocal fold vibration pattern before and after the surgical procedure. In addition, excision of large epithelial lesions can result in residual dysphonia. When the vocal fold epithelium has to be removed, the surgery is limited to the epithelial surface, preserving the subepithelial structures and the vocal ligament. Undoubtedly, we would rather leave an irregular and vibrating margin than a precise and non-vibrating margin. Another problem found by the authors was excessive residual inflammation of vocal folds found one week after the surgery, visualized by laryngoscopy. For marked edema or inflammation, an aggressive treatment with antibiotics, steroids, and oral hydration is early indicated in the postoperative period. There are various factors that cause edema or excessive inflammation, such as persistent cough, rhinosinusitis, bronchitis, allergy and laryngitis by gastroesophageal reflux disease. Woo12 observed that a significant number of patients presented restoration of vibration patterns of vocal folds after aggressive drug therapy. Vocal hygiene measures also contributed to vocal recovery, such as suspension of caffeine and nicotine, which dry the oral mucosa, and use of mucolytic agents.
In our cases, we did not use drugs, but only vocal hygiene measures and voice therapy with good long term results. Maybe the use of postoperative aggressive drug treatment would have provided earlier considerable improvement in vocal quality. Voice therapy has a key role in postoperative rehabilitation of dysphonic patients. It is important to constantly reassess laryngoscopic findings during therapy to optimize results.
Finally, surgical re-exploration is indicated for recurrent or persistent lesions after surgery whose conservative treatment failed.
In our cases, improvement in vocal quality was evident. However, all patients have to be previously informed that recovery to normal vocal patterns sometimes can not be fully achieved.
ConclusionVocal fold polyp is an expressive pathology, common among benign vocal fold mucosa lesions. Treatment is basically surgical and regardless of the modalities and techniques used, a multidisciplinary team is required for successful treatment. The physician should make the diagnosis and assess the best treatment approach for each patient depending on individual characteristics.
Above all, patients' awareness about the limitations of treatment and also about partial recovery of normal vocal pattern are important considerations to avoid misinterpretations and frustrated expectations.
References1. Zargi M, Kambic V, Radsel Z, Acko M. Vocal cord polyps: incidence, histology and pathogenesis. J Laryngol Otol 1981;95:609-618.
2. Woo P, Noordzij JP. Glottal area waveform analysis of benign vocal fold lesions before and after surgery. Ann Otol Rhinol Laryngol 2000;109:441-446.
3. Dikkers FG, Nikkels PGJ. Benign lesions of the vocal folds: histopathology and phonotrauma. Ann Otol Rhinol Laryngol 1995;104:698-703.
4. Gray SD, Hammond E, Hanson DF. Benign pathologic responses of the larynx. Ann Otol Rhinol Laryngol 1995;104:13-18.
5. Loire R, Bouchayer M, Cornut G, Bastian RW. Pathology of benign vocal fold lesions. Ear Nose Throat J 1988;67:357-362.
6. Hirano M, Gould WJ, Lambiase A, Kakita Y. Vibratory behavior of the vocal folds in a case with a unilateral polyp. Folia phoniat 1981;33:275-284.
7. Kojima H, Shinohara K, Tsuji T, Omori K. Videoendoscopic laryngeal surgery. Ann Otol Rhinol Laryngol 2000;109:149-155.
8. Kojima H, Nonomura M, Omori K, Hirano S. Fiberoptic laryngomicrosurgery with stroboscope under local anesthesia. Pract Otol (Kyoto) 1991;84:645-649.
9. Strong MS, Jako GJ. Laser surgery in the larynx: early clinical experience with continuous CO2 laser. Ann Otol Rhinol Laryngol 1972;81:791-798.
10. Shapshay SM, Rebeiz E E, Bohigian RK, Hybels RL. Benign lesions of the larynx: should the laser be used ? Laryngoscope 1990;100:953-957.
11. Remacle M, Lawson G, Watelet JB. Carbon Dioxide laser microsurgery of benign vocal fold lesions: indications, techniques, and results in 251 patients. Ann Otol Rhinol Laryngol 1999;108:156-164.
12. Woo P, Casper J, Colton R, Brewer D. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: a retrospective analysis of 62 patients. Laryngoscope 1994;104:1084-1091.
[1] Resident Physician, Discipline of Otorhinolaryngology, Medical School of ABC.
[2] Instructor, Discipline of Otorhinolaryngology, Medical School of ABC; Doctorate studies under course, Discipline of Otorhinolaryngology, FMUSP.
[3] Faculty Professor, Discipline of Otorhinolaryngology, Medical School of ABC.
Affiliation: Medical School of ABC - Discipline of Otorhinolaryngology.
Address correspondence to: Suzana Boltes Cecatto - Rua São Paulo, 2484
Barcelona - 09541-100 - São Caetano do Sul - SP - E-mail: suzanacecatto@yahoo.com.br


