INTRODUCTIONMiddle ear cholesteatoma is characterized as the presence of keratinized stratified squamous epithelium, retaining a great amount of keratin lamellae inside the cavity, with hyperproliferative and destructive characteristics, which may evolve to complications.
Histologically, cholesteatoma epithelium is similar to the normal skin epidermis, containing the four classical strata of the epidermal tissues (basal, spinosum, granulosum and corneum strata). The epithelium of the cholesteatoma is also called matrix and the subepithelial connective tissue containing collagen and elastic fibers, fibrocytes and inflammatory cells, is called perimatrix of the cholesteatoma 1.
Transmission electron microscopy has enabled detailed assessment of cellular structures and it is now possible to observe protein filaments in the cytoplasm of cells, which are approximately 10mm in diameter, named intermediate filaments. These filaments are part of the cytoskeleton and are divided into 5 different types, each one characterizing a specific tissue2, 3. Among them, the most complex ones are cytokeratins (CKs), present in all epithelial cells. Twenty CKs have been cataloged so far, with molecular weighs that vary from 40 to 68kD and pH between 5 and 8. They are subdivided into acid and base CKs, and an acid CK together with a base form a CK pair, which is the minimum necessary to create an intermediate filament. According to CK patterns expressed by a cell, we can infer the type of epithelium it belongs with (simple or stratified), type of cellular differentiation it follows (esophageal, horny or type of skin), and the stage of cellular proliferation (normal or hyperproliferative), because each pair of CKs is characterized by a specific type of epithelium.
It is known that cholesteatoma has a CK pattern similar to normal epidermis, the external acoustic canal epithelium and epithelial layer of tympanic membrane, containing pairs of CKs 1-2/10 and 5/14. The mucosa of the middle ear expresses different CKs (CKs 8/18 and 19), typical of simple epithelium. It is one further indication that the best theory to explain the etiopathogenesis of acquired cholesteatoma is epithelial migration.
In addition to the CKs described above, there is also the expression of CK16 in suprabasal layers, the matrix of the cholesteatoma, characteristic of hyperproliferative epithelium4, 5, 6. CK 16 does not appear in normal epidermis, except in areas submitted to pressure and friction and in the recovery epithelium of hair follicles7. However, it is always present in hyperproliferative epidermal diseases8, be them benign (psoriasis, vulgar verrucous, contact dermatitis, atopic dermatitis, dermatomyositis, actinic keratosis) or malignant (verrucous carcinoma, squamous and base cell carcinoma). Specific regions such as the tympanic ring and medial and inferior region of the acoustic external canal, together with the ring, also express CK7-9.
Among the substances used as cellular proliferation markers, there is Ki-67, which is an antigen present in the cell nucleus during the multiplication phase (phases late G1, S, G2 and M of cellular cycle). Some authors such as Bujia et al.10 and Mayot et al.11 observed high percentage of positive Ki-67 cells in the cholesteatoma matrix compared to normal epidermis and the percentage is different in different regions of the same case, depending on the variables, such as epithelium thickness, presence of granulation and higher amounts of inflammatory cells in the perimatrix.
The purpose of the present study was to detect, by employing immunohistochemical methods, the presence of CK 16 and Ki-67 in cholesteatoma, correlating morphological variables and checking the differences between cholesteatoma in children and in adults.
MATERIAL AND METHODThe sample of the present study consisted of 31 patients submitted to otologic surgery based on clinical history and physical examination compatible with cholesteatoma chronic otitis media, between 1998 and 2000, from which we collected samples for histology study.
The inclusion of patients in the study followed the criteria below:
- Finding of middle ear cholesteatoma during the surgical procedure;
- Availability of tissue mounted en bloc in paraffin collected from surgical samples, representing the epithelium (cholesteatoma matrix).
The patients operated in this period and included in the study were aged between 4 and 64 years, mean age of 22.16 years and median age of 18 years. Out of 31 studied patients, 21 were male (67.74%) and 10 were female (32.26%). As to age, there were 11 subjects younger than 16 years (children - 35.48%) and 20 who were 16 or older (adults - 64.52%). We selected the parameter of 16 years to subdivide the two age groups based on the studies by Palva et al.12 and Sheehy13.
The material studied was fixed in formol at 10% and processed by histology traditional techniques, with inclusion of paraffin, submitted to sections in rotational microtome, with sections of 3 micrometers thick for histology and immunohistochemical exams. Some sections were submitted to staining with hematoxylin-eosin to analyze the morphological variables. Non-stained sections were placed on slides previously immersed in organosylane adhesive (Sigma, USA), and submitted to immunohistochemical technique through peroxidase in a 3-step procedure, with amplification by the avidine-biotine-peroxidase system, without previous tripsinization. Monoclonal antibodies used were anti-Ki-67 clone Ki-S5 (Dako, Denmark) and keratin 16 (hyperproliferation-related keratin) Ab-1 clone LL025 (Neomarkers, USA).
The morphological aspects analyzed were:
· Atrophy: thinning of cholesteatoma matrix, by reducing size of cells;
· Acanthosis: increased thickness of epithelium, by proliferation of spinosum stratum cells;
· Basal layer hyperplasia with formation of epithelial cones: increase in number of cells of matrix basal layer, forming invaginations into the perimatrix;
· Associated inflammatory process: characterized by intensity of perimatrix permeation by lymphocytes, neutrophils, plasmocytes and macrophages;
· Presence of granulation tissue: characterized by loose connective tissue, rich in neoformed vessels and containing intertwined inflammatory cells.
Variables of the associated inflammatory process and presence of granulation tissue can only be assessed in cases in which there is perimatrix at optical microscopy.
The following variables were also analyzed, using the immunohistochemical method:
· Ki-67 present in the basal layer of the cholesteatoma.
· Ki-67 present in the suprabasal layer of the matrix.
· Ki-67 present in the most apical portion of the matrix (excluding horny layer).
· CK 16 in the suprabasal layers of the matrix.
Both morphological and immunohistochemical variables were assessed in a semi-quantitative and graded fashion, according to severity from 0 to 3, as follows:
0 - absent; 1 - mild; 2 - moderate; 3 - severe.
We performed statistical analysis of data collected using Fisher's exact test, Mann-Whitney non-parametric test and Spearman's correlation coefficient. All tests were performed in bicaudal form, admitting significance level of 5%.
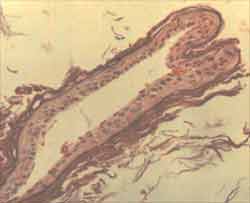
Figure 1. Fragment of cholesteatoma matrix with complete atrophy of epithelium and severe ortokeratosis (ä). There is no representation of perimatrix in this sample. (HE - X 200).
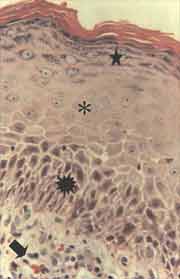
Figure 2. Section of cholesteatoma fragment showing matrix with prominent acanthosis ([), basal layer hyperplasia (ä) and hypergranulosis (i). In the perimatrix we can observe the mixed inflammatory infiltrate (è), with lymphocytes, plasmocytes and hystiocytes in addition to vascular neoformation. (HE - X 400).
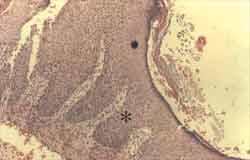
Figure 3. Section of cholesteatoma fragment showing the matrix with severe hyperplasia of basal layer, forming wide epithelial cones ([), which invade the perimatrix. We can also notice acanthosis (ä) in the sample, and the epithelium generates great amount of keratin lamellae. In the perimatrix there is a dense lympho-hystiocytarian inflammatory infiltrate (HE - X 100).
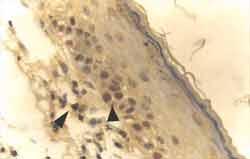
Figure 4. Strongly positive reaction to Ki-67 antibody, staining nuclei of basal layer cells of the matrix of cholesteatoma fragment (ä). The antibody has also stained a lymphocyte in the perimatrix (ä) (IH - X 400).
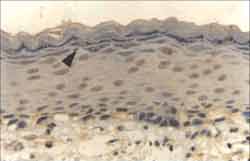
Figure 5. Strongly positive reaction to Ki-67 antibody in the nuclei of basal layer cells, extending to suprabasal layers and also to the apical layer (ä) (IH - X 400).
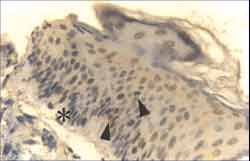
Figure 6. Strongly positive reaction to Ki-67 antibody in the nuclei of basal layer cells of cholesteatoma fragment, extending to suprabasal layers (ä). Note slight hyperplasia of basal layer indicating beginning of epithelial cone formation ([) (IH - X 400).
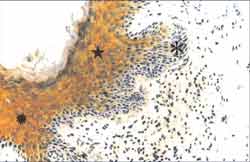
Figure 7. Strongly positive reaction to anti CK-16 antibody in the suprabasal layer of the matrix of a cholesteatoma fragment (i). Note acanthosis (ä) and matrix basal layer hyperplasia, forming wide epithelial cones ([), which invade the perimatrix. (IH - X 200).
As to clinical-surgical assessment, 11 of the 31 studied patients presented some type of complication, such as erosion of the posterior wall of external acoustic canal, fistula of semicircular canals, erosion of mastoid cortical, retroauricular fistula, mastoiditis, meningitis and peripheral facial paralysis. Considering all complications found, the most frequent one was semicircular canal fistula, adding up to 6 cases.
The anatomy pathologic result of the 31 analyzed cases showed heterogeneity in its representation of all studied variables, even in the same case.
In nearly all analyzed fragments, we observed alternation between thinned areas of stratified squamous epithelium and other with predominance of acanthosis. Of the 31 cases studied in the present paper, 5 cases (16.13%) had only atrophy and one case (3.22%) had only acanthosis. In 3 cases (9.68%), we observed balance between the areas of atrophy and acanthosis. In other 22 cases (70.97%), both variables seemed to have different intensities, and there was predominance of atrophy (values equal or greater than 2) in 14 cases (45.16% of the total cases) and predominance of acanthosis (values equal or greater than 2) in 8 cases (25.81% of the total). The distribution of these variables can be seen in Graph 1.
The epithelium originated most of the keratin lamellae. In sites in which the matrix was thicker, we could notice areas with basal layer hyperplasia, forming epithelial cones that invaded the perimatrix. Basal layer hyperplasia with epithelial cones was detected in 41% of the 31 cases, at variable intensities, as shown by Graph 2.
Perimatrix was not present in all cases. Only 22 of them (70.97%) had corium representation (Graph 3), thus, only in these cases the inflammation intensity and granulation tissue could be analyzed. As to inflammation, of the 22 assessable cases, it was present in 20 of them (90.91%), with intensities varying from low to high (Graph 4). Granulation tissue was detected in approximately half of the assessable cases, in mild intensities (Graph 5).
As to immunohistochemical assessment, heterogeneity of reactions continued to be constant. In all samples, Ki-67 was present in the nucleus of basal layer cells, at variable intensities, tending from moderate to severe (Graph 6). In 21 of 31 cases (67.74%), antibody Ki-67 reaction extended to the suprabasal layers (Graph 7), and in 10 cases (32.26%) it was maintained positive up to the most apical layer, right below the horny layer (Graph 8).
As to CK 16, the reactions were predominantly in the cytoplasm of suprabasal cells, also in variable intensities at different sites in the same case. Seven of the 31 cases (22.58%) did not react to anti-CK 16 antibodies (Graph 9).
As to statistical analysis of results, they were positive and significant for correlations of the variables listed below, using Spearman's correlation coefficient:
· apical Ki-67 with CK 16, suprabasal Ki-67 with CK 16 and suprabasal Ki-67with apical Ki-67 (p=0.0160, p=0.0001 and p=0.0001 respectively).
· suprabasal Ki-67 and CK 16 with basal layer hyperplasia with epithelial cone formation (p=0.0051 and p=0.0040 respectively).
· suprabasal CK 16 and Ki-67 with acanthosis (p=0.0001 and p=0.0001 respectively).
As to variables atrophy and acanthosis, we used Spearman's correlation test that showed negative and significant correlation between them (p=0.0001, respectively).
The other defined correlations did not show statistically significant differences between the studied groups (moderate and severe inflammation x suprabasal Ki-67, apical CK16 and presence of complication; complications x basal layer hyperplasia forming epithelial cones; expression of moderate and severe CK 16 in adults and children).
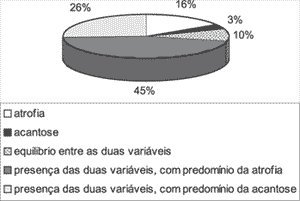
Graph 1. Distribution of atrophy and acanthosis areas in the 31 studied cases.
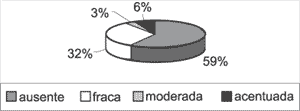
Graph 2. Distribution of variable basal layer hyperplasia forming epithelial cones in the 31 studied cases.
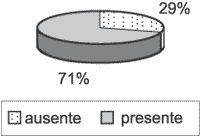
Graph 3. Presence and absence of perimatrix in the 31 studied cases. Only 22 (29%) have perimatrix present.
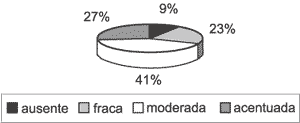
Graph 4. Distribution of associated inflammatory process variable in the 31 studied cases.
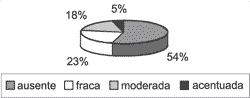
Graph 5. Distribution of granulation tissue in the 31 studied cases.
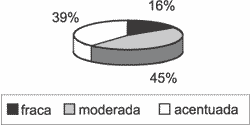
Graph 6. Distribution of variable basal Ki-67 in the 31 studied cases.
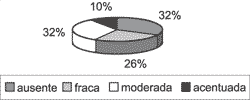
Graph 7. Distribution of variable suprabasal Ki-67 in the 31 studied cases.
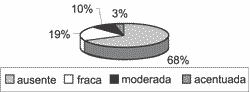
Graph 8. Distribution of variable apical Ki-67 in the 31 studied cases.
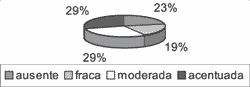
Graph 9. Distribution of variable suprabasal CK 16 in the 31 studied cases.
As to atrophy and acanthosis, in 25 of the 31 studied cases (70.97%) both variables were present in the middle ear of the same patient. In only one of the 31 cases there was no area of atrophy. Atrophy means reduction of cell size owing to loss of cellular substance, with reduction of structural components. They represent a form of adaptation response, on which the cell has reduced functioning, but is not dead. When the sufficient number of cells is involved, all the tissue becomes atrophic. This process can result from various causes, among them: reduction of blood flow, inappropriate nutrition and compression. Since apoptosis can be triggered by the same mechanisms, atrophy can progress up to the point in which cells are damaged and die. Various studies, such as the ones by Albino, Kimmelman, Parisier4, Chae et al.14 and Tomita15, studied apoptosis of cholesteatoma and showed that inducing substances of apoptosis, such as tumor necrosis factor (TNF), beta transforming growth factor (TGF-beta) and p53, have higher levels in cholesteatomas than in normal skin.
By correlating the variables atrophy and acanthosis, there was negative and significant correlation between them, showing that atrophy and acanthosis are inversely proportional. Thus, the greater atrophy is in an area, the smaller is acanthosis, and vice-versa.
To study inflammation, it is necessary to assess the perimatrix, since it is the site in which the inflammatory cells are located. Of the 31 studied cases, the perimatrix was visualized in only 22 cases (70.97%); for this reason, inflammation could only be studied in such cases. There is no way of knowing whether the other 9 cases (29.03%) had inflammation. According to Lim & Saunders1, all cholesteatomas have perimatrix, but it cannot always be visualized by optic microscopy. In some cases, perimatrix is extremely thin and can only be observed by transmission electron microscopy. In such cases, collagen fibers are almost absent, but the crystalline structures are abundant, dispersed in the amorphous material of the perimatrix. These authors believe that reduction in number of collagen fibers is an indication of collagenase activity, an enzyme that destroys collagen fibers and is present in cholesteatoma. Another factor to justify this fact is the pressure exerted by the cholesteatoma content on the matrix and perimatrix, compressing the tissue against the middle ear bone framework. Both the collagenase activity and the pressure on the bone walls of the middle ear are factors that can explain the power of bone erosion the cholesteatoma has. The results of the present study showed that adults had higher amount of perimatrix visible at optic microscopy than children. It is a finding to be further investigated, because if there is in fact more collagenase in children than in adults, it could explain why the cholesteatoma in children is clinically more aggressive than in adults.
As to the present study, there were 22 cases with perimatrix visible at optic microscopy that could be assessed, and inflammatory cells were found in 20 cases, amounting to 90.91%. The inflammatory reaction found was mixed, containing neutrophils, macrophages, plasmocytes and lymphocytes. There was no statistical correlation between the cases with and without inflammation, because practically all cases with perimatrix had inflammatory cells. This finding is in accordance with the clinical observation, since we rarely find a cholesteatoma without an inflammatory process.
As to studied immunohistochemical variables, the results obtained in the present study showed that CK 16 is expressed by cells of suprabasal layer of the cholesteatoma matrix, heterogeneously, and among our 31 studies cases, 7 did not react to anti-CK16 antibody, in a total of 22.58%, similar to the statistics published by Kakoi et al.16, of 25%.
We also analyzed the immunoexpression of Ki-67 antigen, present in the nucleus of cells that are in the multiplication phase. The cellular cycle consists of phases G1, S, G2, and M. Phase G0 is the one in which the cells are acquiescent, at rest, but at any time can get into cycle and start the multiplication process. Phases G1 and G2 are transition stages, phase S is the one in which there is synthesis and duplication of DNA and phase M is the one in which there is the mitosis per se. Ki-67 is present in late G1 phase, S, G2 and M of the cellular cycle, and absent from early G1 and G0 17, 18. It is a widely used antibody to study the growth potential of tumor cells, analyzing the fraction of growth of the tumor, that is, how many cells are in the cellular cycle. Cattoretti et al.19 reported the equivalence between Ki-67 and antibodies MIB 1, MIB 2 and MIB 3, which were produced by using the recombining parts of the nuclear antigen Ki-67. KI-67 is present in the nucleus of some basal cells of the external acoustic canal epithelium, in small percentage. In cholesteatoma, the percentage of positive Ki-67 cells in the basal layer is greater, and it extends to suprabasal layers4, 10, 20-22. As to basal layer of cholesteatoma matrix, the reaction is approximately 2.3 times greater than the one we can find in the external acoustic canal epidermis20, 22.
The results of the present study showed 100% of the cases with Ki-67 in the basal layer. Twenty-one of 31 cases (67.74%) showed positive Ki-67 in suprabasal cells. Ten cases (32.26%) reacted to Ki-67 antibodies also in the superficial layer of the matrix, right below the horny layer (apical Ki-67), being one child (10% of the apical Ki-67 cases) and 9 adults (90%). The difference between presence of apical Ki-67 in adults and in children was statistically significant, and in adults we found more positive apical Ki-67 cells than in the group of children, differently from what we empirically expected when compared to the clinical aggressiveness of cholesteatoma in children and in adults. As to basal and suprabasal Ki-67, there were no differences between the two age groups. Bujia et al.10 also studied MIB 1 relation with cholesteatoma matrix samples in 15 adults and in 20 children and they found a greater average of positive MIB 1 cells in the group of children than in the group of adults, which was a statistically significant difference, the opposite result to our study. Since the authors studied a larger number of children than us (20 and 11 children, respectively), the variable should be further studied with a greater number of subjects, to check if there is really a statistically significant difference. Another factor that should be taken into consideration is that Bujia et al. did not specify up to what age the subjects were considered children.
The correlation between variables showed some statistically significant differences. As to inflammatory process of perimatrix, more than 90% of the cases had some degree of inflammation, cases were subdivided into two groups in which one had absent or mild inflammation (values below 2) and the other had moderate to severe inflammation process (values above 2). There was no correlation between these two groups and the presence of variables apical and suprabasal CK 16 and Ki-67. Therefore, by this study we could infer that inflammation is a variable found in practically all cases of cholesteatoma, but there was no positive correlation between inflammation severity and presence of apical or suprabasal CK 16 or Ki-67, differently from the findings by Sudhoff et al.23, Sudhoff et al.22 and Vennix et al.24.
A very interesting correlation was defined between variable suprabasal Ki-67, apical Ki-67 and suprabasal CK 16. There was positive and statistically significant correlation between these variables. Thus, we can infer that the greater the value of suprabasal KI-67, the greater the amount of CK 16. The greater the apical Ki-67, the greater the amount of CK 16. As a consequence, the greater suprabasal Ki-67, the greater the apical Ki-67. Since CK16 is a characteristic of hyperproliferating epithelium and Ki-67 is an indication of cellular proliferation, these results confirm the hyperproliferative nature of cholesteatoma.
Carrying on with the statistical analysis of the studied variables, we tried to check whether there was correlation between existence of complications and presence of variables suprabasal Ki067, apical Ki-67, CK 16, basal membrane hyperplasia with epithelial cones and inflammation (moderate to severe). There was no association among these complications and the presence of all those variables. It would be interesting to retest these variables in a larger group, because empirically, more aggressive cases, with greater power of bone destruction and progression to complication, should express greater amounts of suprabasal, apical Ki-67 or CK 16. Hoppe, in 199525, has also analyzed the expression of Ki-67 in 9 cases of aggressive cholesteatoma, 30 less aggressive and six recurrence cases. The division between more or less aggressiveness in cases of cholesteatoma was based on intraoperative findings, such as degree of bone erosion and presence of inflammatory process and exuberant granulation tissue. The author did not find statistically significant differences between types of cholesteatoma, but the number of cases about aggressive cholesteatoma was even smaller than ours (nine aggressive cases and 11 cases of complication, respectively).
As to epithelial cones and acanthosis, there was positive and significant correlation between these two variables and expression of suprabasal Ki-67 and suprabasal CK16. Thus, the greater the expression of suprabasal Ki-67 and suprabasal CK16, the greater basal layer hyperplasia and acanthosis of the epithelium. These results show that basal layer hyperplasia forming epithelial cones has a direct correlation with variables that indicate hyperproliferation, in accordance with the studies by Bernal Sprekelsen et al.20, Hoppe25, Mayot et al.11 and Sudhoff et al.22. Moreover, the thicker the matrix, the greater the expression of CK16 and Ki-67, which are in accordance with the results by Bernal Sprekelsen20 and Sudhoff et al.22.
CONCLUSIONSCholesteatoma is a hyperproliferative disease presenting CK16 in the suprabasal layers and Ki-67 nuclear antigen in basal layer, extending to the suprabasal layers and including the apical layer in the matrix, at different intensities and sites of the patients' middle ear.
The greater the concentration of suprabasal and apical Ki-67, the greater the cytokeratin 16 in suprabasal layers of cholesteatoma matrix. The thicker the cholesteatoma matrix and the greater the amount of epithelial cones invading the perimatrix, the higher the expression of cytokeratin 16 and Ki-67 in the suprabasal layers of cholesteatoma matrix. There was no statistically significant difference between cholesteatoma in the two groups (children and adults), concerning variables suprabasal CK16 and Ki-67, but only concerning apical Ki-67, in which we noticed an increase in intensity of this variable in the adults compared to children.
REFERENCES1. Lim DJ & Saunders WH. Acquired cholesteatoma: light and electron microscopic observations. Ann Otol 1972;81:2-12.
2. Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest 1985;52:243-56.
3. Moll R, Franke WW, Schiller DL. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31:11-24.
4. Albino AP, Kimmelman CP, Parisier SC. Cholesteatoma: a molecular and cellular puzzle. Am J Otol 1998;19:7-19.
5. Kim CS & Chung JW. Morphologic and biologic changes of experimentally induced cholesteatoma in mongolian gerbils with anticytokeratin and lectin study. Am J Otol 1999;20:13-8.
6. Pereira CSB. Análise de estudos da expressão das citoqueratinas no colesteatoma adquirido. São Paulo, 1997 (dissertação - mestrado - Faculdade de Ciências Médicas da Santa Casa de São Paulo).
7. Lepercque S, Broekaert D, Van Cauwenberge P. Cytokeratin expression patterns in the human tympanic membrane and external ear canal. Eur Arch Otorhinolaryngol 1993;250:78-81.
8. Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56- kDalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol 1984;98:1397-405.
9. Broekaert D, Coucke P, Leperque S, Ramaekers F, Van Muijen G, Boedts D, Leigh I, Lane B. Immunohistochemical analysis of the cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann Otol Rhinol Laryngol 1992;101:931-8.
10. Bujia J, Holly A, Antolí-Candela F, Tapia MG, Kastenbauer E. Immunobiological Peculiarities of cholesteatoma in children: quantification of epithelial proliferation by MIB1. Laryngoscope 1996;106:865-7.
11. Mayot D, Béné MC, Perrin C ,Faure GC. Restricted expression of Ki-67 in cholesteatoma epithelium. Arch Otolaryngol Head Neck Surg 1993;119:656-8.
12. Palva A, Karma P, Kärjä J. Cholesteatoma in children. Arch Otolaryngol 1977;103:74-7.
13. Sheehy JL. Management of cholesteatoma in children. Adv Oto-Rhino-Laryng 1978;23:58-64.
14. Chae SW, Song JJ, Suh HH, Lim HH, Hwang SJ. Expression patterns of p27Kip1 and Ki-67 in cholesteatoma epithelium. Laryngoscope 2000;110:1898-901.
15. Tomita S. Aspectos moleculares do colesteatoma - imunoexpressão das proteínas controladoras do ciclo celular:p53, bax e bcl-2. São Paulo, 2000 (tese - doutorado - Escola Paulista de Medicina).
16. Kakoi H, Tamagawa Y, Kitamura K, Anniko M, Hiraide F, Kitajima Y. Cytokeratin expression patterns by one- and two- dimensional electrophoresis in pars flaccida cholesteatoma and pars tensa cholesteatoma. Acta Otolaryngol 1995;115:804-10.
17. Brown DC & Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology 1990;17:489-503.
18. Gerdes J, Lemke H, Baisch H, Wacker H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984;133:1710-5.
19. Cattoretti G, Becker MHG, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992;168:357-63.
20. Bernal Sprekelsen M, Ebmeyer J, Buchbinder A, Sudhoff H. Comparative analysis of the proliferative capacity of cholesteatoma. Acta Otorrinolaringol Esp 2000;51:299-307.
21. Ergün S, Zheng X, Carlsöö B. Antigen Expression of epithelial markers, collagen IV and Ki67 in middle ear cholesteatoma. An immunohistochemical study. Acta Otolaryngol (Stockh) 1994;114:295-302.
22. Sudhoff H, Bujia J, Fisseler-Eckhoff A, Holly A, Schulz-Flake C, Hildmann H. Expression of a cell-cycle-associated nuclear antigen (MIB1) in cholesteatoma and auditory meatal skin. Laryngoscope 1995;105:1227-31.
23. Sudhoff H, Borkowski G, Bujia J, Hildmann H, Fisseler-Eckhoff A. Immunhistochemische untersuchungen von mittelohrschleimhautresten im cholesteatom. HNO 1997;45:630-5.
24. Vennix PPCA, Kuijpers W, Peters TA, Tonnaer ELGM, Ramaekers FCS. Keratinocyte differentiation in acquired cholesteatoma and perforated tympanic membranes. Arch Otolaryngol Head Neck Surg 1996;122:825-32.
25. Hoppe F. Untersuchung zum proliferationsverhalten von cholesteatomen. HNO 1995;43:710-5.
[1] Doctorate studies in Otorhinolaryngology under course, Medical School, Santa Casa de São Paulo.
[2] Faculty Professor, Medical School of Jundiaí.
[3] Anatomic Pathologist, Hospital Alemão Oswaldo Cruz.
Address correspondence to: Dra. Celina S. B. Pereira: Rua Sabará, 566 conj. 23
Higienópolis 01239-011 - São Paulo - SP
E-mail: cpereira@dialdata.com.br
Article selected as one of the top 10 studies in Otology, awarded with a citation and as the best study in the supra-specialty.


