INTRODUCTIONHospital infection (HI) is defined as an infection related to hospitalization or medical procedures carried out in a hospital setting acquired by the patient after admission that manifests during hospitalization or up to 72 hours after discharge1.
Studies indicate that the most common type of ICU HI is ventilator-associated pneumonia (VAP). Other frequent causes of HI are urinary tract infections associated to the use of indwelling bladder catheter and catheter-related bloodstream infections. These three causes account for over 80% of ICU HI cases2-4.
Infective rhinosinusitis (IRS) is usually not mentioned in large international and national studies on HI, and remains as a topic discussed only in epidemiology studies on infections on severe patients2,3,5. Nonetheless, several papers have described IRS as one of the main causes of fever in ICUs2,6-8.
The diagnosis of IRS in ICU patients can be challenging for otorhinolaryngologists. In this group of sedated immune-depressed patients, the classic signs of rhinosinusitis are mostly absent9,10. Clinical findings are nonspecific, and patients have fever and are often diagnosed with leukocytosis3,8,11,12. Once other possible foci of infection are ruled out, ENTs are called to assess these critical patients with fever of unknown origin (FUO), leukocytosis, and signs of disorder on imaging tests.
Paranasal sinus CT scans and nasal endoscopy are the most important noninvasive tests used to assess ICU patients. Presence of air-fluid level, complete sinus opacification or mucosal thickening greater than 6 mm combined with purulent secretion in the middle meatus visualized during endoscopic examination are deemed as important signs in the diagnosis of sinusitis13.
Patients with FUO and radiologic signs of rhinosinusitis are first advised to remove all nasal devices6,14-16. Additionally, topical nasal vasoconstrictors are administered for at least 72 hours before they are offered an invasive procedure17.
If the patient fails to respond to these initial measures, it is recommended that the infected secretion be drained from the paranasal sinus8,12,14,15. This procedure offers the possibility of confirming the diagnosis of IRS and improving the patient's overall condition.
Maxillary sinus puncture is considered the gold standard to diagnose IRS in ICU patients8,12,14,15. Besides offering clinical samples used to identify the causing agent of infective syndromes, it may also serve as an important tool in the treatment of this group of patients6,12,15. Authors have described success rates of approximately 70% in managing fever after removing infected secretion from the maxillary sinus14. The great advantage of this procedure is that it can be performed at the bedside in the ICU, thus reducing the risk of adverse effects connected to taking the patients to the OR18.
Critical rhinosinusitis patients with fever refractory to the procedure are traditionally referred to functional endoscopic sinus surgery. They are given general anesthesia to allow for the removal of all infected secretion, excision of local inflammatory tissue, and ventilation of the involved paranasal sinuses16,17,19.
However, ICU patients are at a higher risk for surgery, as they are immunocompromised and unstable from the hemodynamic and metabolic standpoints. Additionally, these individuals often present relevant coagulation disorders. Therefore, surgery should only be offered as a last resort for these patients.
This study aims to assess the role of maxillary sinus puncture at the bedside in the diagnosis and treatment of patients with infective rhinosinusitis admitted to the ICU of a high complexity university hospital.
MATERIALS AND METHODSThis retrospective study looks into the ENT assessment of ICU patients with radiologic signs of rhinosinusitis with fever of unknown origin (axillary temperatures equal to or greater than 37.8ºC) on ventilation staying at a high complexity hospital.
FOU was defined as fever not linked to identified disease or cases in which patients failed to respond despite being given proper therapy for a previously diagnosed condition, according to the protocol in effect at this service.
The patients and procedures described in this study were cared for and performed by a team of physicians made up by two ENT resident medical doctors (second and third year residents) and one ENT specialized in rhinology who led the team.
This study and the informed consent form presented to the patients were approved by the Ethics Committee of our institution and granted permit # 1158/09.
The patients enrolled in this study had to meet specific inclusion and exclusion criteria (consecutive allocation).
The inclusion criteria were:
time of hospitalization greater than 48 hours;use of invasive ventilation;presence of fever of unknown origin (axillary temperature > 37.8ºC);maxillary sinus involvement verified in CT scans;fever persisting despite the administration of xylometazoline twice a day for 72 hours;absence of nasal devices for at least 72 hours;paranasal sinus puncture positive for infection (presence of purulent secretion and/or evidences of bacterial growth).
The exclusion criteria were:
facial trauma with direct involvement of the paranasal sinuses;deviated septum touching the nasal lateral wall, increasing the complexity of the puncture procedure when done through the inferior meatus;polyps, antrochoanal polyps, and other nasal lesions.
Three tools were used to assess patient findings:
A- Nose endoscopy: the patients were examined with a 3.1 mm flexible fiberscope; presence of purulent secretion in the middle meatus was deemed as a sign suggestive of IRS.
B- Paranasal sinus CT scans: presence of complete opacification, air-fluid level, or mucosal thickening
> 6 mm.
C- Paranasal sinus puncture: positive diagnosis was defined when purulent secretion and/or evidence of bacterial growth were found20,21.
Patients underwent maxillary sinus puncture via the inferior meatus to treat IRS7,12 (Figure 1). IRS cases refractory to puncture were treated through functional endoscopic sinus surgery (FESS); patients were placed under general anesthesia to have their involved sinuses meticulously drained16,17,22,23. Proper antibiotics were administered by the intensive care team and infectologists, as verified by the analysis done on secretion aspirate cultures.
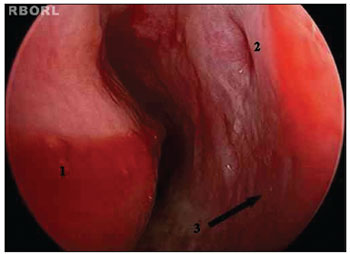
Figure 1. Left inferior meatus. 1: Inferior nasal concha; 2: Hasner's valve; 3: Site of puncture on the lateral wall of the left inferior meatus.
Onset of defervescence (reduction on the number of daily peaks of fever) within 48 to 72 hours16,17 or abolition of fever within five days of the procedure17 where used to characterize the success of the puncture procedure. The same criteria was used to assess the success of FESS16,17,22,23.
Fever abatement was used as a parameter to assess intervention effectiveness. The patients who failed to evolve favorably even after surgery were reassessed clinically and radiologically through control paranasal sinus CT scans. After discussing the case with the patient and the ICU team, a new procedure was offered whenever it was deemed necessary.
Sinus aspirates and sinus washout contents were collected and sent in sterile containers for culturing and antibiogram analysis.
The variables considered for analysis were patient gender, age, diagnosis on ICU admission, involved paranasal sinus (radiological signs), alterations on physical examination (presence or absence of purulent secretion in the middle meatus), clinical improvement after puncture, culture results, occurrence of adverse events related to the procedures, need for surgery, improved clinical outcome with surgery.
This paper used a statistical significance level of 0.05 (5%). Statistical analysis was done using the test for equality of two proportions. The test for equality of two proportions is a non-parametric test (used in low sampling) compares the proportion of answers of two variables and whether their levels are statistically significant.
Software products SPSS V16, Minitab 15 and Excel Office 2007 were used in statistical analysis.
RESULTSThis study enrolled 27 critical patients assessed by the rhinology service physicians of a university hospital between March of 2007 and September of 2010.
Patient data can be seen on Table 1. The study flowchart is presented in Figure 2.
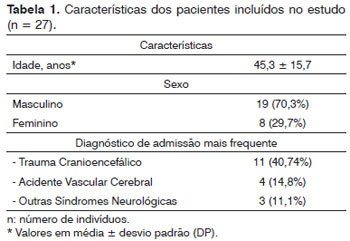
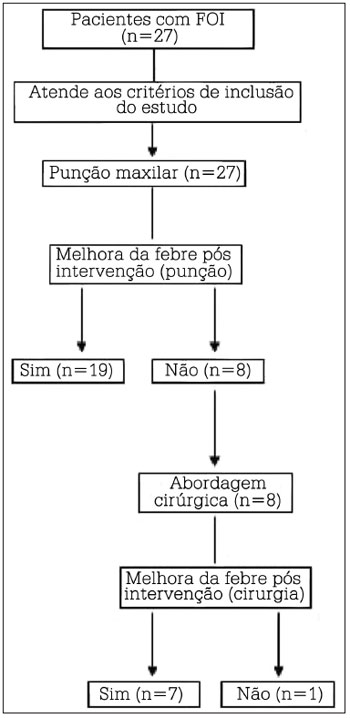
Figure 2. Study organizational flowchart. n = number of subjects.
Fifty-four nasal cavities were analyzed through CT scans. All patients had radiological signs of unilateral or bilateral maxillary and sphenoidal rhinosinusitis (Figure 3). Two patients (7.4%) had all paranasal sinuses involved as seen in CT scans.
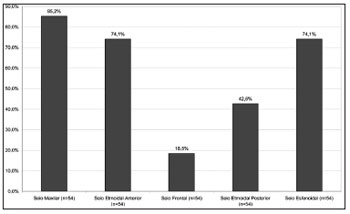
Figure 3. Distribution of paranasal sinuses with radiological sings of rhinosinusitis. n = number of nasal cavities.
Twenty-six nasal cavities (13 patients) were examined through endoscopy for the presence of alterations suggestive of rhinosinusitis in the middle meatus (Table 2).
Glycopeptide antibiotics were the most used antimicrobial agents in this study (Figure 4). The most common drug combinations were glycopeptides and carbapenems (42.6%), and glycopeptides and fourth generation cephalosporins (38.1%).
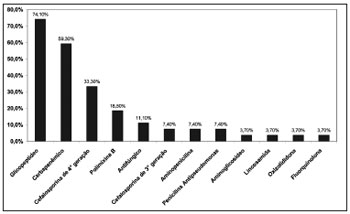
Figure 4. Distribution of antibiotics administered to patients diagnosed with infective rhinosinusitis.
Forty-six maxillary sinus puncture procedures were performed, based on the alterations found on paranasal CT scans. Postoperative clinical improvement was observed in 70.4% of the patients (Table 3).
No complications were recorded in the maxillary sinus puncture procedures.
Microbiological analysis was done for 26 patients, and at least one agent responsible for IRS was found (Figure 5).
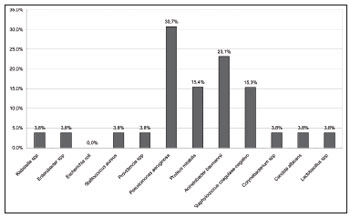
Figure 5. Distribution of microbiological analyses of maxillary sinus aspirates.
Eight patients (29.6%) underwent FESS. After surgery, seven patients (87.5%) improved from fever and one (12.5%) did not (Table 4). The cause of fever in this patient was attributed to the relapse of a previously diagnosed digestive system tumor.
DISCUSSIONIRS is one of the causes for fever of unknown origin in ICU patients and, as such, must be thoroughly investigated2,3,6,7. This infective syndrome increases patient morbidity and may be associated with difficulty managing some diseases such as VAP.
Prevalence rates were higher among male patients in this study. Head trauma was the main cause for hospitalization in the studied population. These findings were similar to those seen in the literature9,13,16,24. This population comprises a group of severe patients who often require prolonged hospitalization. Additionally, they are often on ventilation for longer periods of time (hyperventilation) to control intracranial pressure levels16. Consequently, some authors have described lower levels of consciousness, scores under 7 in the Glasgow coma scale, and/or head trauma as risk factor for IRS25.
The same imaging criteria described in the literature were used in this study: air-fluid level, complete opacification, or mucosal thickening greater than 6 mm24,26,27. In general terms, the most frequently involved paranasal sinuses were the maxillary and the sphenoidal, as seen in 85.2% and 74.1% of the nasal cavities respectively. The ethmoid and frontal sinuses also presented significant rates of involvement. Some authors have described a positive correlation between sinus disease and length of intubation20,28, i.e., the longer the period the patient is on the ventilator the greater the number of involved paranasal sinuses. All patients in this study were intubated for over two weeks, which possibly led to the high rates of involvement observed.
One of the most characteristic diagnostic signs of rhinosinusitis is purulent secretion in the middle meatus. Only 30.7% of the nasal cavities of the critical patients enrolled in this study were blocked. Kountakis et al.13 e Skoulas et al.24 described rates of 25.2% and 40.3% respectively. These low rates indicate that the classic endoscopic signs of rhinosinusitis may be absent in most ICU patients, given the altered levels of immune response of this population9,10.
ICU patients with hospital infection were being treated empirically with broad specter antibiotics at the time of their ENT examination. Initial therapy for FUO individuals must consider the endemic hospital bacterial flora. Glycopeptides, carbapenems, and fourth generation cephalosporins were the most frequently used drugs. The drugs were changed by the ICU medical staff and infectologists based on the results of sinus aspirate cultures. There is no consensus in the literature as to the duration of antibacterial administration, but a minimum of seven days of drug therapy seems to be necessary7,15.
Some authors believe that antibacterial therapy alone is not enough to treat IRS patients. Studies have shown that ICU patients with IRS still had fever even when the concentration of antibiotic drugs in their sinus aspirates was high enough to attain the minimum inhibitory level to eliminate bacteria. A complex set of pathologic variables can be used to explain this event, among which is the formation of a biofilm in the nasal mucosa preventing antibacterial drugs from reaching their targets. These authors concluded that drainage of the infected secretion is required to treat IRS20.
Patients with FUO and radiological signs of rhinosinusitis are advised to remove their nasal devices as part of their initial assessment. Various papers have described nasal devices as one of the main risk factors for the development of IRS8,14-16. These devices may press against the ostiomeatal complex and mechanically block the sinus ostium. Additionally, trauma produced by this tube may trigger an inflammatory response in the nasal mucosa, mucosal edema, increased secretion, and alterations in the property of mucous fluid29.
Other factors have been associated with increased nasal congestion in these individuals: prolonged periods in dorsal decubitus, high central venous pressure, and positive pressure ventilation. These factors increase jugular venous pressure, thus congesting the vessels in the nasal mucosa29.
Therefore, patients are required to remove their nasal devices and use nasal topical vasoconstrictors for 72 hours before undergoing invasive procedures8,17,25. These measures may reverse the inflammatory process in some individuals and facilitate the drainage of sinus secretions.
Many studies have indicated maxillary sinus puncture as the standard procedure used in to diagnose IRS8,12,22,26,30. This procedure may also be a relevant tool in the treatment of critical patients with IRS8,12,22,26,30. This study revealed that 70.4% of the patients improved from their fever episodes after the procedure. This outcome is similar to that published by Salord et al.14 and Rouby et al.26. In the study by Caplan & Hoyt25, 58.8% of the patients improved from fever, while Ramadan et al.15 published improvement rates of 83%.
No complications were recorded in the maxillary sinus puncture procedure.
Community-acquired rhinosinusitis is usually caused by
Streptococcus pneumoniae and Haemophilus influenzae. Hospital rhinosinusitis has been related to colonization by endogenous bacteria and pathogens exogenous to the ICU environment29. The most frequently microorganisms observed in our study were
Pseudomonas aeruginosa (30.7%),
Acinetobacter baumannii (23.1%),
Proteus mirabilis (15.4%) and coagulase-negative
Staphylococcus (15.3%). The results of microbiologic analysis indicate that the changes in the nasal and systemic immune profile of these critical patients may enable pathogens to colonize the patients' airways and trigger the onset of IRS. Similar findings were previously reported in the literature8,21,27,30-32.
Poorly cared for pharynxes, oral cavities and teeth may play an important role in the colonization of paranasal sinuses by bacterial pathogens29. Gastric protection and nasoenteral tubes often used in severe patients also favor the spread of bacteria in the stomach29.
Previously used nasal devices and the injured mucosa around them may offer a good bed for the development of pathogens. Bacteria populations may grow in these areas and form biofilms.
P. aeruginosas and
S. aureus have mucus and respiratory epithelial cell receptors. As
A. baumannii, they also produce glycocalyx, which adheres to polyvinyl chloride, a material present in endotracheal tubes7. In this study,
P. aeruginosas and
A. baumannii were the most frequently observed pathogens. Therefore, the presence of biofilm in the nasal mucosa may trigger ongoing inflammatory response and sustained fever episodes even after the infected secretion has been removed from the patients' sinuses. The high rates of involvement of posterior paranasal sinuses, more specifically of the sphenoidal sinus19,20, may be a factor in the therapeutical failure of the maxillary sinus puncture procedure15. These factors may account for the clinical failure of the puncture procedure in these patients.
In this study, FESS was indicated for patients whose puncture procedures failed to resolve their IRS. In all, 87.5% of the patients improved from fever after FESS. Pádua et al.19 also described a high success rate (82%) with surgery.
CONCLUSIONMaxillary sinus puncture at the bedside enabled the identification of at least one significant pathogen responsible for hospital infective rhinosinusitis. The most frequently observed pathogens were
Pseudomonas aeruginosa (30.4%) and
Acinetobacter baumannii (23.1%). The procedure proved to be a relevant therapeutical tool, and showed success rates of 70.4% in managing patient fever.
REFERENCES1. Brasil. Ministério da Saúde. Portaria 2616, de 12 de Maio de 1998. Diário Oficial da União. 13 de Maio 1998. Secção I, p.133.
2. Marik PE. Fever in the ICU. Chest. 2000;117(3):855-69.
3. Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120(6):2059-93.
4. O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-49.
5. de Assis DB, Madalosso G, Ferreira SA, Yassuda YY. Análise dos dados de infecção hospitalar do estado de São Paulo, 2008. Bol Epidemiol Paul. 2009;6(1):16-29.
6. Deutschman CS, Wilton P, Sinow J, Dibbell D Jr, Konstantinides FN, Cerra FB. Paranasal sinusitis associated with nasotracheal intubation: a frequently unrecognized and treatable source of sepsis. Crit Care Med. 1986;14(2):111-4.
7. Bert F, Lambert-Zechovsky N. Sinusitis in mechanically ventilated patients and its role in the pathogenesis of nosocomial pneumonia. Eur J Clin Microbiol Infect Dis. 1996;15(7):533-44.
8. Van Zanten AR, Dixon JM, Nipshagen MD, de Bree R, Girbes AR, Polderman KH. Hospital-acquired sinusitis is a common cause of fever of unknown origin in orotracheally intubated critically ill patients. Crit Care. 2005;9(5):R583-90.
9. Geiss HK. Nosocomial sinusitis. Intensive Care Med. 1999;25(10):1037-9.
10. Kortbus MJ, Lee KC. Sinusitis and fever of unknown origin. Otolaryngol Clin North Am. 2004;37(2):339-46.
11. Hansen M, Poulsen MR, Bendixen DK, Hartmann-Andersen F. Incidence of sinusitis in patients with nasotracheal intubation. Br J Anaesth. 1988;61(2):231-2.
12. Linden BE, Aguilar EA, Allen SJ. Sinusitis in the nasotracheally intubated patient. Arch Otolaryngol Head Neck Surg. 1988;114(8):860-1.
13. Kountakis SE, Burke L, Rafie JJ, Bassichis B, Maillard AA, Stiernberg CM. Sinusitis in the intensive care unit patient. Otolaryngol Head Neck Surg. 1997;117(4):362-6.
14. Salord F, Gaussorgues P, Marti-Flich J, Sirodot M, Allimant C, Lyonnet D, et al. Nosocomial maxillary sinusitis during mechanical ventilation: a prospective comparison of orotracheal versus the nasotracheal route for intubation. Intensive Care Med. 1990;16(6):390-3.
15. Ramadan HH, El Solh AA. An update on otolaryngology in critical care. Am J Respir Crit Care. 2004;169(12):1273-7.
16. Deutschman CS, Wilton PB, Sinow J, Thienprasit P, Konstantinides FN, Cerra FB. Paranasal sinusitis: a common complication of nasotracheal intubation in neurosurgical patients. Neurosurgery. 1985;17(2):296-9.
17. Humphrey MA, Simpson GT, Grindlinger GA. Clinical characteristics of nosocomial sinusitis. Ann Otol Rhinol Laryngol. 1987;96(6):687-90.
18. Fanara B, Manzon C, Barbot O, Desmettre T, Capellier G. Recommendations for the intra-hospital transport of critically ill patients. Crit Care. 2010;14(3):R87.
19. Pádua FG, Bezerra TF, Voegels RL, Bento RF. The efficacy of functional endoscopic sinus surgery in the evolution of fever of unknown origin in ICU patients. Acta Otolaryngol. 2011;131(2):166-72.
20. Fassoulaki A, Pamouktsoglou P. Prolonged nasotracheal intubation and its association with inflammation of paranasal sinuses. Anesth Analg. 1989;69(1):50-2.
21. Bert F, Lambert-Zechovsky N. Microbiology of nosocomial sinusitis in intensive care unit patients. J Infect. 1995;31(1):5-8.
22. Borman KR, Brown PM, Mezera KK, Jhaveri H. Occult fever in surgical intensive care unit patients is seldom caused by sinusitis. Am J Surg. 1992;164(5):412-5.
23. Bach A, Boehrer H, Schmidt H, Geiss HK. Nosocomial sinusitis in ventilated patients. Nasotracheal versus orotracheal intubation. Anaesthesia. 1992;47(4):335-9.
24. Skoulas IG, Helidonis E, Kountakis SE. Evaluation of sinusitis in the intensive care unit patient. Otolaryngol Head Neck Surg. 2003;128(4):503-9.
25. Caplan ES, Hoyt NJ. Nosocomial sinusitis. JAMA. 1982;247(5):639-41.
26. Rouby JJ, Laurent P, Gosnach M, Cambau E, Lamas G, Zouaoui A, et al. Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am J Respir Crit Care Med. 1994;150(3):776-83.
27. Pneumatikos I, Konstantonis D, Tsagaris I, Theodorou V, Vretzakis G, Danielides V, et al. Prevention of nosocomial maxillary sinusitisin the ICU: the effects of topically applied alpha-adrenergic agonists and corticosteroids. Intensive Care Med. 2006;32(4):532-7.
28. Payne SC, Benninger MS. Progression of sinus disease in the intubated patient. Am J Rhinol. 2006;20(2):230-4.
29. Riga M, Danielidis V, Pneumatikos I. Rhinosinusitis in the intensive care unit patients: a review of the possible underlying mechanisms and proposals for the investigation of their potential role in functional treatment interventions. J Crit Care. 2010;25(1):171.e9-14.
30. Souweine B, Mom T, Traore O, Aublet-Cuvelier B, Bret L, Sirot J, et al. Ventilator-associated sinusitis microbiological results of sinus aspirates in patients on antibiotics. Anesthesiology. 2000;93(5):1255-60.
31. George DL, Falk PS, Umberto Meduri G, Leeper KV Jr, Wunderink RG, Steere EL, et al. Nosocomial sinusitis in patients in the medical intensive care unit: a prospective epidemiological study. Clin Infect Dis. 1998;27(3):463-70.
32. Balsalobre Filho LL, Vieira FMJ, Stefanini R, Cavalcante R, Santos RD, Gregório LC. Nosocomial sinusitis in an intensive care unit: a microbiological study. Braz J Otorhinolaryngol. 2011;77(1):102-6.
1. MD, ENT (MSc student in the ENT graduate program at UNIFESP-EPM).
2. MD (ENT Resident at UNIFESP-EPM).
3. MD, ENT (Rhinology fellow at UNIFESP-EPM).
4. MSc in ENT at UNIFESP-EPM (PhD student in the ENT graduate program at UNIFESP-EPM).
5. PhD in ENT at UNIFESP-EPM (Assisting Physician in the course of Rhinology at UNIFESP-EPM).
6. Associate Professor at UNIFESP-EPM (Head of the Rhinology Department at UNIFESP-EPM).
UNIFESP-EPM.
Send correspondence to:
José Arruda Mendes Neto
Rua Padre Machado, 778, apto 53
São Paulo - SP. CEP: 04127-001
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on August 25, 2011.
Accepted on April 22, 2012. cod. 8747.


