INTRODUCTIONHead and neck carcinoma is the fifth most frequent cancers; its incidence worldwide has been estimated at 50,000 new cases each year1,2. The estimated rate for oral cancer in Brazil during 2010 was 14,120 new cases (10,330 men and 3,790 women)3. Most of these epithelial tumors are classified as head and neck squamous cell carcinoma (HNSCC); the anatomical sites and occurrences in this group are the mouth (40%), the pharynx (15%), and the larynx (25%)2,4-6.
About two thirds of patients with these diseases present at advanced stages in which regional lymph nodes are generally involved. Distance metastases are present in 10% of patients7. The treatment varies depending on the stage of the disease; according to published data, about 60% to 65% of head and neck cancer patients may be cured by surgery and/or radiotherapy. Patients at initial stages of the disease (I and II) are treated by a single form of therapy (surgery or radiotherapy), while patients at more advanced stages (III and IV) require a combined approach, such as surgery and radiotherapy or chemotherapy8.
This cancer affects mostly males at more advanced age groups; the mean age at diagnosis is 60 years7,9. However, the incidence of cancer involving the base of tongue and the tonsils has increased in individuals aged below 45 years; this has been attributed to an increased prevalence of HPV infection, which is a contributing factor for this disease in developing countries10-11.
The main and well-established risk factors for this disease are smoking and alcohol abuse; these habits jointly multiply the risk of cancer, especially in the mouth and pharynx12. The reason for this is that cigarettes contain about 4,700 substances, of which at least 50 are carcinogenic. Frequent consumption of alcohol renders epithelial cells unable to form a protective barrier against external agents, thereby facilitating the action of cigarette carcinogens, which form DNA adducts that are not recognized in DNA replication processes13,14.
Hashibe et al.12 published a study showing that alcohol abuse, independently from smoking, elevated significantly the risk of oropharyngeal, hypopharyngeal, and laryngeal cancer in individuals that had never smoked. Alcohol abuse may also cause nutritional deficiencies because of altered intestinal absorption, and may alter important metabolic pathways, such as the folate metabolism, which is involved in cell methylation reactions. Consequently, gene methylation with a potential role in carcinogenesis may be compromised15.
Studies have suggested that poor oral hygiene is associated with a higher risk of head and neck cancer. Periodontal disease because of poor oral hygiene may result in infection; inflammation mediators - such as cytokines - are released and reactions against inflammation occur, which may foster the development of cancer16. Loss of teeth may also facilitate the onset of mouth cancer, as the oral flora may become abnormal, and nitrite and nitrate reduction and production of acetaldehyde may occur, leading to formation of DNA adducts14,17.
Published papers have shows that a diet rich in whole cereals, fruit, and vegetables, and with few processed foods, together with a healthy life style, may confer protection against DNA oxidative damage. These foods contain micronutrients - vitamins B, C, E, carotenoids, flavonoids, and other - that possess antioxidant and anticarcinogenic activity, which reduces the risk of oral cancer18-21.
Folate deficiency in the body - a vitamin that may be found in fruit and vegetables - has been associated with an increased risk of several types of cancer, including head and neck cancer20,22-26. This micronutrient is involved in DNA synthesis, repair, and methylation22,27.
OBJECTIVE AND METHODSThe purpose of this study was to carry out a review of the literature to present the results of studies that have assessed the modulation of polymorphisms involved in folate metabolism and the risk of head and neck cancer.
FOLATE METABOLISMFolate is involved in forming methyl (CH3) groups during a carbon interconversion in the intermediate metabolism of S-adenosilmethionine (SAM), which is a methyl group donator in cell methylation reactions26,28,29. DNA methylation consists of transferring methyl groups to position 5 of cytosine residues that are located on cytosine-guanine dinucleotides (CpG); this occurs in reactions catalyzed by proteins named DNA methyltransferases30. This epigenetic DNA modification has several functional roles, such as controlling gene expression, stabilizing chromatin structure, and maintaining genomic stability23,26,29,30-34.
There are three mechanisms by which altered folate metabolism may contribute to carcinogenesis: (1) DNA hypomethylation and subsequent proto-oncogene activation26,35; (2) uracil misincorporation during DNA synthesis, leading to genomic instability26,35,36; and (3) increased cytosine deamination in DNA methylation sites26,36.
Abnormal folate levels due to genetic polymorphisms in its metabolic pathway are associated with altered DNA methylation, synthesis and repair; adequate folate levels are essential for the biosynthesis of purines and pyrimidines, which are needed in these biological processes37-45.
Figure 1 shows the enzymes that are involved in folate metabolism. First, folate is converted or reduced into physiological folate by the dihydrofolate reductase (DHFR) enzyme. The serine hydroxymethyltransferase enzyme (SHMT) catalyzes a reversible reaction of THF into 5,10-MTHF; this enzyme is key in maintaining and regulating the homeostasis of folate concentration and intracellular methyl groups. It requires vitamin B6 and has a significant role in protein and DNA synthesis and in methylation reactions involving nucleic acids46,47.
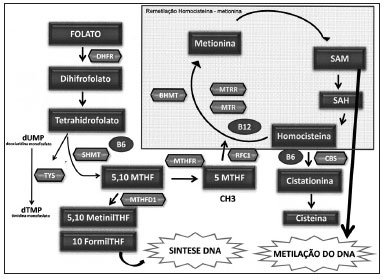
Figure 1. Main enzymes involved in the folate metabolism. Folate metabolism - DHF: Dihydrofolate; THF- Tetrahydrofolate; DHFR: Dihydrofolate reductase; SHMT: Serine hydroxymethyltransferase; TYS: Thymidylate synthase; MTHFD1: Methylenetetrahydrofolate dehydrogenesase 1; MTHFR: Methylene tetrahydrofolate reductase; MTR: methionine synthase; MTRR: Methionine synthase reductase; BHMT: Betaine-homocysteine methyltransferase; CBS: Cystathionine beta synthase; RFC1: Reduced folate carrier 1; SAM: S S-adenosilmethyonine; SAH: S- adenosylhomocysteine; dUMP: Deoxyuridine monophosfate; dTMP: Thymidine monophosfate.
The methylenetetrahydrofolate reductase (MTHFR) enzyme catalyzes the conversion of 5,10 methylenetetrahydrofolate into 5- methyltetrahydrofolate (5-MTHFR), which is the main circulating form of folate; it operates as a methyl group donor for the remethylation of homocysteine (Hcy) into methionine. This reaction is catalyzed by the methionine synthase enzyme (MTR), which requires vitamin B12 (methylcobalamin) as a cofactor, and which forms SAM. The methionine synthase reductase (MTRR) enzyme maintains the active state of the MTR enzyme. Following the methylation of Hcy, the resulting methionine is condensed with adenosine triphosphate (ATP), which results in S-adenosilmethionine (SAM). Next, in a demethylation reaction, S-adenosylhomocysteine (SAH) is formed and then hydrolyzed to release adenosine and Hcy, thereby completing the cycle48.
Hcy methylation replenished the stocks of SAM when methionine reaches lower levels. The betaine homocysteine methyltransferase (BHMT) enzyme catalyzes the conversion of Hcy into methionine by an alternative remethylation pathway in which the betaine amino acid donates the methyl groups49-51. When the Hcy remethylation pathway - which is catalyzed by the folate-dependent MTR enzyme - is altered by genetic or environmental factors, the BHMT enzyme has a crucial role in Hcy homeostasis52.
Also involved is the cystathionine b-synthase (CβS) enzyme (also requiring vitamin B
6), which has a crucial role in folate metabolism; it converts Hcy into cystathionine in the transsulfuration pathway53,54.
THF is recovered during the methionine regeneration cycle, after the methyl group (5-metil -THF) is donated to homocysteine. THF may be used directly - in another pathway - in the synthesis of thymidylate synthase (TS), which converts deoxyuridine monophosphate (dUMP) into thymidine monophosphate (dTMP) by using the 10-formyl-THF for DNA synthesis. In this reaction, 5,10 methylene THF is the substrate of thymidylate55.
The methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) enzyme catalyzes the oxidation of 5,10-methylene-THF into 5,10-methynyl-THF, which is then converted into 10-formyl-THF (Stevens
et al., 2007). These three reactions are involved in the interconversion of THF carbon-1 derivates, which are the substrates for synthesizing methionine, thymidylate, and purines56.
Another enzyme is the reduced folate carrier 1 (RFC1) enzyme, which is found on the membrane of intestinal mucosa cells, and which is involved in folate absorption. It does so by transporting 5-MTHF into several types of cells, and is an important determinant of folate concentration within cells48.
GENETIC POLYMORPHISMS INVOLVED IN FOLATE METABOLISM AND HEAD AND NECK CANCER
The MTHFR C677T polymorphismThis polymorphism is associated with decreased enzyme activity by limiting conversion of 5,10 methylenetetrahydrofolate into 5-MTHFR, which is the folate form required for DNA methylation reactions57. A in vitro study has shown that the heterozygous 677CT genotype is associated with a 40% decrease in enzyme activity, while the polymorphic homozygote 677TT genotype is associated with a 70% decrease in enzyme activity58.
Additionally, the polymorphic homozygote genotype is associated with lower folate levels and higher homocysteine levels in blood plasma59-61; thus, reduced plasma folate levels may lead to hypomethylation of DNA and cancer62.
As far as we know, eight studies have assessed the association of this polymorphism with head and neck cancer37-44. Of these, only Reljic et al.'s41, Vairaktaris et al.'s40, and Solomon et al.'s43 papers have confirmed an association of the MTHFR C677T polymorphism with a risk of head and neck cancer (Chart 1).
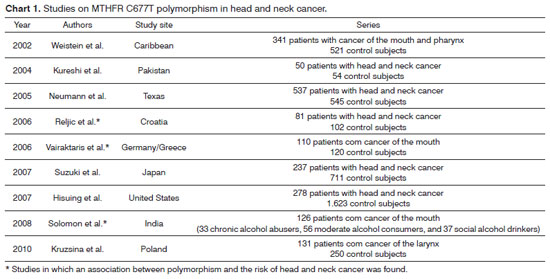
Reljic et al41. conducted a case-control study of 81 patients with head and neck cancer and 102 subjects without a history of cancer among a Croatian population and found that the 677TT genotype decreases the risk of this disease. On the other hand, Vairaktaris et al.40 studied 110 subjects with mouth cancer and 102 cancer-free individuals among Germans and Greeks and found that the 677CT genotype was associated with an increased risk of this cancer. Solomon et al.43 assessed 126 individuals who were alcohol abusers (33 chronic and significant consumers of alcohol, 56 moderate consumers of alcohol, and 37 social drinkers) and who had mouth cancer and found that the 677TT genotype was associated with the group of chronic and significant alcohol abusers and the group of moderate consumers of alcohol (to a lesser degree).
The qMTHFR A1298C polymorphismThis variant has also been associated in vitro with decreased enzyme activity, although to a lesser degree compared to the MTHFR C677T polylmorphism63. A clear biological relevance of this polymorphism remains unclear and results so far are inconsistent62,64,65.
Data on the risk of head and neck cancer in relation to the
MTHFR A1298C polymorphism is contradictory. Suzuki et al.'s42 case-control study of 237 Japanese patients with head and neck cancer and 711 cancer-free individuals and Kruzsina et al.'s44 study of 131 Polish subjects with laryngeal cancer and 250 cancer-free Polish individuals found no association between this variant and the risk of head and neck carcinoma.
Neumann et al.'s39 study of 537 patients with head and neck cancer and 545 control subjects in Texas showed that individuals with the 1298AC or 1298CC genotypes had a 35% lower risk of head and neck cancer. However, this study showed that the risk of HNSCC was higher in individuals with the three polymorphic alleles (
MTHFR 677T,
MTHFR 1298C, and
MTHFR 1793A) compared to subjects with one or two polymorphic alleles.
The MTR A2756G polymorphismAs far as we know, there are no in vitro studies that have assessed the activity of the MTR enzyme in the presence of the
MTR A2756G polymorphism. Data on changes in Hcy and folate levels are contradictory66-71. A few authors have shown that individuals with the polymorphic homozygote
MTR 2756GG genotype present low Hcy and high folate levels68-71. On the other hand, Li et al.66 showed that Hcy levels are high if this variant is present, while Ma et al.67 showed that this polymorphism does not change Hcy levels.
Studies of DNA methylation have shown that the
MTR 2756AG or GG genotypes decrease the formation of SAM, which results in DNA hypomethylation72. Other studies have also shown that there is a relation between the
MTR 2756GG genotype and DNA hypomethylation in colorectal, breast, lung, and cervix cancers72-75.
Three studies have demonstrated the association between this polymorphism and head and neck cancer. Zhang et al.75 conducted a case-control study in Texas of 721 patients with head and neck cancer and 1,234 individuals with no history of cancer and found that the
MTR 2756AG or GG genotypes increased the risk of cancer. Kruzsina et al.'s44 study of 131 Polish subjects with laryngeal cancer and 250 controls also showed that these genotypes (
MTR 2756GG or AG) were associated with this tumor type. Our group studied 236 Brazilian patients with head and neck cancer and 469 controls and found that the
MTR 2756GG genotype and the
MTR 2756G allele were associated with an increased risk of HNSCC45. On the other hand, Suzuki et al.'s42 study of 237 Japanese patients with head and neck cancer and 711 controls revealed no association between this polymorphism and head and neck cancer.
The MTRR A66G polymorphismStudies have shown that this variant generates an enzyme with low affinity for the MTR enzyme76. Gaughan et al.'s77 study showed that individuals with the
MTRR 66GG genotype had low levels of Hcy in the blood plasma compared with individuals having the
MTRR 66AA genotype. This effect, however, was not observed in other studies78,79.
Few studies have investigated an association of the
MTRR A66G polymorphism and the risk of head and neck cancer. Suzuki et al.42 showed that this variant is not associated with a risk for head and neck cancer, but these authors also found an interaction between alcohol abuse and the
MTRR A66G polymorphism in a Japanese population. Zhang et al.75 showed that individuals with the homozygous wild genotype (
MTRR 66AA) are at a lower risk for head and neck cancer, confirming that the A allele is protective.
The RFC1 A80G polymorphismThe
RFC1 gene is involved in intracellular folate transport; it causes 5-MTHFR to be absorbed and transported into several cell types. The
RFC1 A80G polymorphism may be involved in carcinogenesis by altering the concentration of plasmatic Hcy and folate, which in turn are associated with DNA methylation and repair. However, the exact biological mechanism of this polymorphism is not clear48,80-83.
Only our group evaluated the
RFC1 A80G variant and its risk for head and neck cancer; we confirmed that the
RFC1 80AG or 80AA genotypes were associated with an increased risk for these cancers, especially in males aged over 50 years that smoked cigarettes84.
Other polymorphisms of the folate metabolismOnly one study assessed the effect of the
MTHFD1 G1958A polymorphism in head and neck cancer; it found no associated risk for this disese44. Associations between head and neck cancer and the
CBS 844ins68,
BHMT G742A,
SHMT C1420T,
TC2 A67G, and
TC2 C776G polymorphisms, which are also involved in folate metabolism, have not been studied; two of these polymorphisms have been associated with other types of cancer85-89.
CONCLUSIONThe
MTHFR C677T,
MTHFR A1298C,
MTR A2756G,
MTRR A66G, and
RFC1 A80G polymorphisms appear to modulate the risk of head and neck cancer. However, because of contradictory findings, studies of different populations are needed to clarify the role of these polymorphisms in the etiology of head and neck cancer.
REFERENCES1. Chen YJ, Chang JT, Liao CT, Wang HM, Yen TC, Chiu CC, et al. Head and neck cancer in the betel quid chewing area: recent advances in molecular carcinogenesis. Cancer Sci. 2008;99(8):1507-14.
2. Marcu LG and Yeoh. A review of risk factors and genetic alterations in head and neck carcinogenesis and implications for current and future approaches to treatment. J Cancer Res Clin Oncol. 2009;135(10):1303-14.
3. http: www.inca.gov.br/estimativa/2010/index.asp
4. Lothaire P, de Azambuja E, Dequanter D, Lalami Y, Sotiriou C, Andry G, et al. Molecular markers of head and neck squamous cell carcinoma: promising signs in need of prospective evaluation. Head Neck. 2006;28(3):256-69.
5. Ragin CCR, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on a human papillomavirus. J Den Res. 2007;86(2):104-14.
6. Salzwimmer M. Best supportive care in HNSCC. Wien Med Wochenschr. 2008;158(9-10):278-82.
7. Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute 2006.
8. Licitra L, Locati LD, Bossi P. Optimizing approaches to head and neck cancer. Metastatic head and neck cancer: new options. Ann Oncol. 2008;19(7):200-3.
9. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108.
10. D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-56.
11. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612-9.
12. Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer. Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777-89.
13. Rubin, H. Synergistic mechanisms in carcinogenesis by polyciclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis. 2001;22(12):1903-30.
14. Choi S, Myers JN. Molecular Pathogenesis of Oral Squamous Cell Carcinoma: Implications for Therapy. J Dent Res. 2008;87(1):14-32.
15. Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7(2):149-56.
16. Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124(4):823-35.
17. Abnet CC, Qiao Y-L, Dawsey SM, Dong Z-W, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34(2):467-74.
18. Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126-34.
19. Marchioni DML, Fisberg RM, Góis Filho JF, Kowalski LP, Carvalho MB, Abrahão M, et al. Dietary patterns and risk of oral cancer: a case-control study in São Paulo, Brazil. Rev Saúde Pública. 2007;41(1):19-26.
20. Garavello W, Lucenteforte E, Bosetti C, Talamini R, Levi F, Tavani A, Franceschi S, Negri E, La Vecchia C. Diet diversity and the risk of laryngeal cancer: A case-control study from Italy and Switzerland. Oral Oncol. 2009;45(1):85-9.
21. Prado RP, dos Santos BF, Pinto CLS, Assis KRC, Salvadori DMF, Ladeira MSP. Influence of diet on oxidative DNA damage, uracil misincorporation and DNA repair capability. Mutagenesis. 2010;25(5):483-7.
22. Suzuki T, Wakai K, Matsuo K, Hirose K, Ito H, Kuriki K, et al. Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci. 2006;97(8):760-7.
23. Xu WH, Shrubsole MJ, Xiang YB, Cai Q, Zhao GM, Ruan ZX, et al. Dietary folate intake, MTHFR genetic polymorphisms, and the risk of endometrial cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2007;16(2):281-7.
24. Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control. 2008;19(10):1161-70.
25. Garcia-Crespo D, Knock E, Jabado N, Rozen R. Intestinal neoplasia induced by low dietary folate is associated with altered tumor expression profiles and decreased apoptosis in mouse normal intestine. J Nutr. 2009;139(3):488-94.
26. Linhart HG, Troen A, Bell GW, Cantu E, Chao W, Moran E, et al. Folate Deficiency induces genomic uracil misincorporation and hypomethylation but does not increase DNA point mutations. Gastroenterology. 2009;136(1):227-35.e3.
27. Duthie SJ. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis. 2011;34(1)101-9.
28. Bailey LB. Folate, methyl-related nutrients, alcohol, and the MTHFR 677C-T polymorphism affect cancer risk: intake recommendation. Am Soc Nutrl Sci. 2003;133(11 Suppl 1):3748S-53S.
29. Charasson V, Hillaire-Buys D, Solassol I, Laurand-Quancard A, Pinguet F, Morvan VL, et al. Involvement of gene polymorphisms of the folate pathway enzymes in gene expression and anticancer drug sensitivity using the NCI-60 panel as a model. Eur J Cancer. 2009;45(13):2391-401.
30. DAlessio AC, Szyf M. Epigenetic tête-à-tête: the bilateral relationship between chromatin modifications and DNA methylation. Biochem Cell Biol. 2006;84(4):463-76.
31. Tuck-Muller CM, Narayan A, Tsien F, Smeets DF, Sawyer J, Fiala ES, et al. DNA hypomethylation and unusual chromosome instability in cell lines from ICF syndrome patients. Cytogenet Cell Genet. 2000;89(1-2):121-8
32. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415-28.
33. Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88(5):899-910.
34. Sciandrello G, Caradonna F, Mauro M, Barbata G. Arsenic-induced DNA hypomethylation affects chromosomal instability in mammalian cells. Carcinogenesis. 2004;25(3):413-7.
35. Johanning GL, Heimburger DC, Piyathilake CJ. DNA methylation and diet in cancer. J Nutr. 2002;132(12):3814S-18S.
36. Kane, MA. The role of folates in squamous cell carcinoma of the head and neck. Cancer Detect Prev. 2005;29(1):46-53.
37. Weinstein SJ, Gridley G, Harty LC, Diehl SR, Brown LM, Winn DM, et al. Folate intake, serum homocysteine and methylenetetrahydrofolate reductase (MTHFR) C677T genotype are not associated with oral cancer risk in Puerto Rico. J Nutr. 2002;132(4):762-7.
38. Kureshi N, Ghaffar S, Siddiqui S, Salahuddin I, Frossard PM. Head and neck cancer susceptibility: a genetic marker in the methylenetetrahydrofolate reductase gene. ORL J Otorhinolaryngol Relat Spec. 2004;66(5):241-5.
39. Neumann AS, Lyons HJ, Shen H, Liu Z, Shi Q, Sturgis EM, et al. Methylenetetrahydrofolate reductase polymorphisms and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Int J Cancer. 2005;115(1):131-6.
40. Vairaktaris E, Yapijakis C, Kessler P, Vylliotis A, Ries J, Wiltfang J, et al. Methylenetetrahydrofolate reductase polymorphism and minor increase of risk for oral cancer. J Cancer Res Clin Oncol. 2006;132(4):219-22.
41. Reljic A, Simundic AM, Topic E, Nikolac N, Justinic D, Stefanovic M. The methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and cancer risk: the Croatian case-control study. Clin Biochem. 2007;40(13-14):981-5.
42. Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Wakai K, Hirose K, et al. One-carbon metabolism-related gene polymorphisms and risk of head and neck squamous cell carcinoma: case-control study. Cancer Sci. 2007;98(9):1439-46.
43. Solomon PR, Selvam GS, Shanmugam G. Polymorphism in ADH and MTHFR genes in oral squamous cell carcinoma of Indians. Oral Dis. 2008;14(7):633-9.
44. Kruszyna Ł, Lianeri M, Rydzanicz M, Gajecka M, Szyfter K, Jagodziński PP. Polymorphic variants of folate metabolism genes and the risk of laryngeal cancer. Mol Biol Rep. 2010;37(1):241-7.
45. Galbiatti ALS, Ruiz MT, Biselli-Chicote PM, Raposo LS, Maniglia JV, Pavarino-Bertelli EC, Goloni-Bertollo EM. 5-Methyltetrahydrofolate-homocysteine methyltransferase gene polymorphism (MTR) and risk of head and neck cancer. Braz J Med Biol Res. 2010;43(5):445-50.
46. Scheer JB, Mackey AD and Gregory JF. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr. 2005;135(2):233-8.
47. Niclot S, Pruvot Q, Besson C, Savoy D, Macintyre E, Salles G, et al. Implication of the folate-methionine metabolism pathways in susceptibility to follicular lymphomas. Blood. 2006;108(1):278-85.
48. Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol. 2000;32(4):385-9.
49. Morin I, Platt R, Weisberg I, Sabbaghian N, Wu Q, Garrow TA, Rozen R. Common variant in betaine-homocysteine methyltransferase (BHMT) and risk for spina bifida. Am J Med Genet. 2003;119A(2):172-6.
50. Mason JB, Choi SW. Effects of alcohol on folate metabolism: implications for Carcinogenesis. Alcohol. 2005;35(3):235-41.
51. Ueland PM Holm PI, Hustad S. A key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43(10):1069-75.
52. Weisberg IS, Park E, Ballman KV, Berger P, Nunn M, Suh DS, et al. Investigations of a common genetic variant in betaine-homocysteine methyltransferase (BHMT) in coronary artery disease. Atherosclerosis. 2003;167(2):205-14.
53. Haddad R, Mendes MA, Hoehr NF, Eberlin MN. Amino acid quantitation in aqueous matrices via trap and release membrane introduction mass spectrometry: homocysteine in human plasma. Analyst. 2001;126(8):1212-5.
54. Födinger M, Dierkes J, Skoupy S, Röhrer C, Hagen W, Puttinger H, et al. Effect of glutamate carboxypeptidase ii and reduced folate carrier polymorphisms on folate and total homocysteine concentrations in dialysis patients. J Am Soc Nephrol. 2003;14(5):1314-9.
55. Baluz K, Carmo MGT, Rosas G. The role of folic acid on oncologic prevention and intervention: review. Rev Bras Cancerol. 2002;48(4):597-60.
56. Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71(5):1207-15.
57. Leclerc D, Campeau E, Goyette P, Adjalla CE, Christensen B, Ross M, et al. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet. 1996;5(12):1867-74.
58. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64(3):169-72.
59. Molloy AM, Daly S, Mills JL, Kirke PN, Whitehead AS, Ramsbottom D, et al. Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: implications for folate intake recommendations. Lancet 1997;349(9065):1591-3.
60. Girelli D, Friso S, Trabetti E, Olivieri O, Russo C, Pessotto R, et al. Methylenetetrahydrofolate reductase C677T mutation, plasma homocysteine, and folate in subjects from northern Italy with or without angiographically documented severe coronary atherosclerotic disease: evidence for an important genetic-environmental interaction. Blood. 1998;91(11):4158-63.
61. McNulty H, McKinley MC, Wilson B, McPartlin J, Strain JJ, Weir DG, et al. Impaired functioning of thermolabile methylenetetrahydrofolate reductase is dependent on riboflavin status: implications for riboflavin requirements. Am J Clin Nutr. 2002;76(2):436-41.
62. Friso S, Girelli D, Trabetti E, Olivieri O, Guarini P, Pignatti PF, et al. The MTHFR 1298A.C polymorphism and genomic DNA methylation in human lymphocytes. Cancer Epidemiol. Biomarkers Prev. 2005;14(4):938-43.
63. Lievers KJ, Boers GH, Verhoef P, den Heijer M, Kluijtmans LA, van der Put NM, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med. 2001;79(9):522-8.
64. Chango A, Boisson F, Barbé F, Quilliot D, Droesch S, Pfister M, et al. The effect of 677C-.T and 1298A-.C mutations on plasma homocysteine and 5,10-methylenetetrahydrofolate reductase activity in healthy subjects. Br J Nutr. 2000;83(6):593-6.
65. Narayanan S, McConnell J, Little J, Sharp L, Piyathilake CJ, Powers H, et al. Associations between two common variants C677T and A1298C in the methylenetetrahydrofolate reductase gene and measures of folate metabolism and DNA stability (strand breaks, misincorporated uracil, and DNA methylation status) in human lymphocytes in vivo. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1436-43.
66. Li YN, Gulati S, Baker PJ, Brody LC, Banerjee R, Kruger WD. Cloning, mapping and RNA analysis of the human methionine synthase gene. Hum Mol Genet. 1996;5(12):1851-8.
67. Ma J, Stampfer MJ, Christensen B, Giovannucci E, Hunter DJ, Chen J, et al. A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8(9):825-9.
68. Harmon DL, Shields DC, Woodside JV, McMaster D, Yarnell JW, Young IS, et al. Methionine synthase D919G polymorphism significant but modest determinant of circulating homocysteine concentrations. Genet Epidemiol. 1999;17(4):298-309.
69. Silaste ML, Rantala M, Sampi M, Alfthan G, Aro A, Kesäniemi YA. Polymorphisms of key enzymes in homocysteine metabolism affect diet responsiveness of plasma homocysteine in healthy women. J Nutr. 2001;131(10):2643-7.
70. Chen J, Stampfer MJ, Ma J, Selhub J, Malinow MR, Hennekens CH, et al. Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis. 2001;154(3):667-72.
71. Dekou V, Gudnason V, Hawe E, Miller GJ, Stansbie D, Humphries SE. Gene environment and gene-gene interaction in the determination of plasma homocysteine levels in healthy middle-aged men. Thromb Haemost. 2001;85(1):67-74.
72. Paz MF, Avila S, Fraga MF, Pollan M, Capella G, Peinado MA, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res. 2002;62(15):4519-24.
73. Fang JY, Xiao SD. Folic acid, polymorphism of methyl-group metabolism genes, and DNA methylation in relation to GI carcinogenesis. J Gastroenterol. 2003;38(9):821-9.
74. Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22(22):4632-42.
75. Zhang Z, Shi Q, Liu Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1188-93.
76. Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human me-thionine synthase reductase. Biochemistry. 2002;41(45):13378-85.
77. Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, Yarnell JW, et al. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001;157(2):451-6.
78. Geisel J, Zimbelmann I, Schorr H, Knapp JP, Bodis M, Hübner U, et al. Genetic defects as important factors for moderate hyperhomocysteinemia. Clin Chem Lab Med. 2001;39(8):698-704.
79. O'Leary VB, Parle-McDermott A, Molloy AM, Kirke PN, Johnson Z, Conley M, et al. MTRR and MTHFR polymorphism: link to Down syndrome? Am J Med Genet. 2002;107(2):151-5.
80. Eklof V, Van GB, Hultdin J, Johansson I, Hallmans G, Palmqvist R. The reduced folate carrier (RFC1) 80G > A and folate hydrolase 1 (FOLH1) 1561C > T polymorphisms and the risk of colorectal cancer: a nested case-referent study. Scand J Clin Lab Invest. 2008;68(5):393-401.
81. DeVos L, Chanson A, Liu Z, Ciappio ED, Parnell LD, Mason JB, et al. Associations between single nucleotide polymorphisms in folate uptake and metabolizing 497 genes with blood folate, homocysteine, and DNA uracil concentrations. Am J Clin Nutr. 2008;88(4):1149-58.
82. Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2008;26(1):111-28.
83. Stanisławska-Sachadyn A, Mitchell LE, Woodside JV, Buckley PT, Kealey C, Young IS, et al. The reduced folate carrier (SLC19A1) c.80G[A polymorphism is associated with red cell folate concentrations among women. Ann Hum Genet. 2009;73(Pt 5):484-91.
84. Galbiatti AL, Ruiz MT, Rezende Pinto D, Raposo LS, Maniglia JV, Pavarino- Bertelli EC, et al. A80G polymorphism of reduced folate carrier 1 (RFC1) gene and head and neck squamous cell carcinoma etiology in Brazilian population. Mol Biol Rep. 2011;38(2):1071-8.
85. Wang Y, Guo W, He Y, Chen Z, Wen D, Zhang X, et al. Association of MTHFR C677T and SHMT1 C1420T with susceptibility to ESCC and GCA in a high incident region of Northern China. Cancer Causes Control. 2007;18(2):143-52.
86. Jonge R, Tissing WJE, Hooijberg JH, Jansen G, Kaspers GJL, Lindemans J, et al. Polymorphisms in folate-related genes and risk of pediatric acute lymphoblastic leukemia. Blood. 2009;113(10):2284-9.
87. Guerreiro CS, Carmona B, Gonçalves S, Carolino E, Fidalgo P, Brito M, et al. Risk of colorectal cancer associated with the C677T polymorphism in 5,10-methylenetetrahydrofolate reductase in Portuguese patients depends on the intake of methyl-donor nutrients. Am J Clin Nutr. 2008;88(5):1413-8.
88. Ott N, Geddert H, Sarbia M. Polymorphisms in methionine synthase (A2756G) and cystathionine -synthase (844ins68) and susceptibility to carcinomas of the upper gastrointestinal tract. J Cancer Res Clin Oncol. 2008;134(3):405-10.
89. Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, Seifried A. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control. 2002;13(3):239-48.
1. Biologist (Master's degree student in Health Science - Research Unit on Genetics and Molecular Biology (Unidade de Pesquisa em Genética e Biologia Molecular or UPGEM).
2. Doctoral degree in Health Science (Adjunct professor - Triangulo Mineiro Federal University (Universidade Federal do Triângulo Mineiro or UFTM).
3. Medical doctor, adjunct professor (Adjunct professor - Sao Jose do Rio Preto Medical School (Faculdade de Medicina de São José do Rio Preto or FAMERP).
4. Medical doctor (Master's degree student in Health Science).
5. Adjunct professor of medical and human genetics (Adjunct professor - Sao Jose do Rio Preto Medical School (Faculdade de Medicina de São José do Rio Preto or FAMERP).
6. Adjunct professor on medical and human genetics (Adjunct professor).
FAMERP - Faculdade de Medicina de São José do Rio Preto (Sao Jose do Rio Preto Medical School).
Send correspondence to:
Faculdade de Medicina de São José do Rio Preto ¬ FAMERP
Av. Brigadeiro Faria Lima, 5416 - Vila São Pedro
CEP: 15090-000. São José do Rio Preto - SP
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on August 10, 2010.
Accepted on September 18, 2010. cod. 7260


