INTRODUCTIONHearing loss is one of the most common sensorial disorders. A clinically significant hearing loss may affect 1-3 for every 1000 low-risk newborns1,2; and among newborns in the ICU this rate can reach 2% to 4%3,4.
After analyzing the literature, COMUSA (Multidisciplinary Committee on Hearing Health), made up by otolaryngologists, pediatricians, speech and hearing therapists, and other professionals, made 21 recommendations for early identification, diagnosis and treatment of newborns and infants with hearing impairment5.
In the present study we tried to analyze the demographic and clinical characteristics of the newborns who participated in the Neonatal Hearing Screening Program in a secondary level maternity, identifying the level of failure and the occurrence of hearing loss and its association with the following variables: gestational age, birth weight and neonatal clinical occurrences.
MATERIALS AND METHODSThis was a retrospective study in which we collected the results from the Neonatal Hearing Screening Program implemented in the Neonatal Unit of a Municipal Hospital located in the north of the city of São Paulo, SP. The annual number of live births, seen in the Neonatal Unit in this hospital is around 1,500; with rates of low-birth weight (birth weight <2,500g) of 11%; very low birth weight (birth weight <1,500g) rate of 1.6% and of extreme low birth weight (birth weight <1000g) rate of 0.8%.
In the study, we included the newborns whose hearing screenings were done by the author, during 20 months, between February of 2004 and December of 2006.
This project was submitted to and approved by the Ethics in Research Committee under protocol # 1332/06.
The Neonatal Hearing Screening (NHS) was carried out by means of Transient Stimulus Evoked Otoacoustic Emissions (TEOE) and the Cochleo-eyelid Reflex (CER) by means of an agogô musical instrument (large campanula) at 100 dBSPL of intensity.
TEOE was carried out in both ears using the ILO EchocheckT system, a portable device which uses click stimuli involving frequency bands between 1,500 Hz and 3,800 Hz. The click is presented at an intensity of 75 to 83 dBpeSPS. The response was considered positive (pass) when the otoacoustic emissions captured were 6 dB higher than the noise.
CER happens in 100% of normal hearing children and its lack suggests bilateral hearing loss or central disorder. The study is carried out with an intense sound stimulus and the response is considered present when there is a contraction of the eye's orbicular muscle, seen by eyelid movement.
The newborn who failed the screening was submitted to a complete audiological evaluation for diagnostic purposes. The assessment was based on the study of the TEOE, acoustic immittance measures obtained by the AZ7 immittance meter with a 226 hertz probe for the tympanometric curve, behavioral assessment and brainstem Hearing evoked potential (BAEP), with the Navigator Pro-Biologic Systems Corp
® device with a click-type stimulus.
The children who had risk indicators for hearing impairment, even those who passed the hearing screening, were referred to Hearing development follow up.
The hearing loss was classified as conductive when associated with middle or external ear disorders; or sensorineural, associated with cochlear disorders classified as mild to profound degree6, or retrocochlear, because of a hearing neuropathy or neural conduction changes in the brainstem Hearing pathways.
As far as the demographic characteristics of the children included in the study are concerned, we observed the following data: newborn gender, birth weight, gestational age and Apgar score.
Concerning hearing impairment risk analysis, we collected the following variables: consanguinity, congenital malformations, perinatal asphyxia, hyperbilirubinemia, peri-intraventricular hemorrhage, meningitis, seizures, need for mechanical ventilation, use of ototoxic medication, congenital infections such as syphilis, rubella, toxoplasmosis, cytomegalovirus and human immunodeficiency virus (HIV) diagnosed during gestation or in the neonatal period, smoking, alcohol drinking or the use of illegal drugs during pregnancy; family with hearing loss, diagnosis or suspicion of genetic syndromes made by the pediatrician and/or geneticist, duration of neonatal ICU stay.
We analyzed demographic and clinical characteristics of the newborns included in this study and the rate of failure in the screening, the occurrence of hearing loss and an association between failure in the test and gestational age, birth weight and the main neonatal complications.
The results obtained were described as mean and standard deviation of the numerical variables and frequency for the categorical ones. The investigation concerning the factors associated with otoacoustic emission test failure was carried out by means of a univariate analysis, using the chi-squared or Fisher's test, considering
p<0.05 as significant.
RESULTSDuring the study, 4,593 children were born at the maternity of this hospital. The NHS was carried out in 1,805 newborns (39.3%), 1,615 (89.5%) were born at term, 146 (8.1%) were preterm with birth weight higher than 1,500g and 44 (2.4%) preterm with birth weight lower than 1,500g.
Figure 1 represents the number and percentage of children who participated in the different stages of the Newborn Hearing Screening Program carried out in the present study.
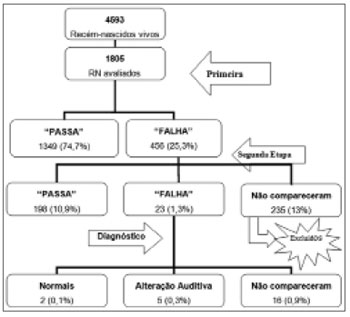
Figure 1. Number and percentage of children considered in the Newborn Hearing Screening Program of the Vereador José Storópolli Hospital.
Of the 1,805 newborn evaluated, 235 did not come for the second stage of the NHS program; thus, only 1,570 newborns were evaluated.
The 235 newborns who did not return, were compared to the 1,570 newborns of the study in relation to gestational age, birth weight and risk indicators, with the goal of checking for similarities between the groups for statistical purposes.
The newborns who completed the second stage of the NHS had similar characteristics in relation to gestational age (38.8±2.2 versus 38.7±2.1 weeks,
p=0.821) and birth weight (3,107±600 versus 3,169±587 grams,
p=0.140), when compared to the ones who did not return.
Thus, we concluded that the children who did not return for an assessment at the second stage were similar to the ones who continued in the NHS program, except for the greater frequency of peri-intraventricular hemorrhage (1.7% versus 0.4%,
p=0.031*).
The sample studied was made up of 1,570 newborns, 790 (50.3%) females and 780 (49.7%) males; 1,401 (89.2%) born at term and 169 (10.8%) were premature.
The children without TEOE and/or CER were considered as failure in this study. Therefore, the failure rate in this study's sample was 26 (1.7%). Of these, nine (0.6%) had one or more risk indicators for hearing loss.
The mean gestational age and birth weight of the children in relation to the NHS are depicted on Table 1.
In regards of risk indicators for hearing impairment, 1,349 (85.9%) newborns, of the 1,570 included in the present study, did not have any risk indicator; and 221 (14.1%) had one or more risk indicators.
In order to assess the failure rate at NHS in relation to the variables: gender, prematurity, ear tested, stay in newborn ICU, risk factors for hearing impairment, we considered the 1,570 newborns who made up the series investigated in this study.
The failure rate was not related to the child's gender (
p=0.223) and ear side (
p=1.000). There was a greater failure rate among preterm newborns (4.1%) in relation to those who were born at term (1.4%);
p=0.017.
In order to analyze failures in relation to the gestational age and birth weight, the children were broken down into three groups:
At term Group (TG): at term newborns with birth weight equal to or higher than 1,500g. Preterm Group (PT >1,500g): premature babies with birth weight equal to or higher than 1,500g. Preterm with very low birth weight Group (PTMBP): premature babies with birth weight lower than 1,500g.
The rate of NHS failures in the PT MBP (15.0%) group was higher than that from the GT (1.2%) and PT >1,500g (1.6%) groups,
p<0.001.
In order to analyze the influence of risk indicators concerning hearing impairment, the sample was broken down according to the number of risk factors presented (from one to five).
Table 2 depicts the failure rate in the neonatal hearing screening in relation to the number of risk indicators.After employing Fisher's exact test, we noticed a statistically significant association between NHS failure and the number of risks. Failures happened less often in children with a maximum of one risk indicator, when compared to those who had two or more risk indicators.
We analyzed 15 risk indicators for HL. Table 3 depicts the statistical analysis and p-values for each risk indicator associated with the failure in neonatal hearing screening, which are depicted on Table 3.
Hearing loss index according to demographic variables and risk indicators After diagnostic assessment, two children (0.1%) had normal results, which were considered false-positives.16 (1%) children did not show up for their return visit and eight (0.5%) had a hearing impairment diagnosis - four being sensorineural hearing loss (0.25%), one conductive hearing loss caused by craniofacial malformation (0.06%); and three children with suspected retrocochlear disorder (0.2%).
The hearing impairment frequency analysis was similar for both genders (
p=0.625) and it was significantly higher in pre-term babies (3.6% vs. 0.2%,
p<0.001), when compared to their term newborn counterparts. Among premature babies, such disorder was more frequency among those of very low birth weight, when compared to those which birth weight higher than 1,500g (12.8% vs. 0.8%,
p<0.001).
We noticed a higher occurrence of hearing disorders in newborns who had one or more risk indicators (Table 4). Nonetheless, it is worth stressing that one child diagnosed with hearing loss did not have any risk indicator.
Table 5 depicts the risk indicators associated with hearing disorders (Table 5). In this analysis we took off the 16 newborns who did not complete the diagnosis process; thus, we analyzed 1,554 children, from whom 1,546 did not have any hearing disorder and eight had a hearing disorder.
There was a statistically significant association between hearing disorders and the risk indicators: family history of hearing loss, craniofacial malformation, use of mechanical ventilation, use of ototoxic drugs, periintraventricular hemorrhage, ICU stay for more than 48 hours and very low birth weight.
DISCUSSIONNewborn Hearing Screening provides for the early detection of hearing disorders, thus enabling intervention before six months of age7.
A hearing health program must bear four stages: detection or hearing screening, audiological diagnosis, hearing aid fitting and the intervention of an audiologist - expert in educational audiology7.
Newborn Hearing Screening is considered a process, and not an event, which provides parents and children a follow up, from pre-screening instructions all the way to the treatment and follow up of the child diagnosed with the hearing loss and the child's family8. Nevertheless, there are still many challenges to be faced, such as: Implementation of a Newborn Hearing Screening Program with the help of maternities - both administratively and also considering the professionals involved in newborn care; effective follow up of those babies who failed the screening process or those bearing risk indicators for hearing disorder. Thus, we can reduce the large number of babies who fail to report back and thus missing the opportunity for a late diagnosis.
Another big challenge is the proper intervention and selection of hearing aids, besides speech and hearing therapies to promote proper language development8.
Only 39.3% of the 4,593 babies born during the study period participated in the Newborn Hearing Screening Program. This rate of coverage was lower than 95% of what was recommended to consider for an effective Newborn Hearing Screening Program3,4.
National and international studies report NHS coverage varying between 41.6% and 99.2%9-13.
In order to guarantee a hearing screening for all newborns, it would have to be performed every day of the week by a trained team concerning the equipment used as well as the protocol to be followed. Moreover, the NHS program should include pediatricians, otolaryngologists and expert audiologists14. Facts which did not happen in the present study, because the NHS was done in only two periods per week and by one professional only.
Of the screenings carried out in the present study, 74.7% had a "passing" result in the first stage of the screening program.
The rate of failure in the NHS (25.3%) for newborns with and without risk indicators for hearing impairment was higher than those in some studies present in the literature10,13,15. In 2007, the
Joint Committee on Infant Hearing (JCIH), recommended that failures in the first stage should not surpass 10%. Such result may be explained by the age in which the first NHS was carried out, since most of the children were assessed within 48 hours of life, before being discharged from the hospital.
Despite the possibility of capturing TOAEs already after 24 hours of life, it is also known that middle ear effusion is also very common in the first 48 hours of life16.
Moreover, factors such as an excess of environment noise or routine procedures which make the child more restless (bath, handling for an exam, drug administration), the external auditory meatus being obstructed by vernix or by wax may contribute to the high rates of retests in NHS programs17.
The high rate of evasion found in the present study and also described in the literature is still a major challenge for audiology professionals9,12,18. The socioeconomical conditions of the population seen could be one of the factors associated with the high rate of newborn evasion in the second stage of the NHS. The high rate of evasion in the diagnostic stage could also be explained by the difficulty in access, having seen that the diagnosis was made in another hospital, far from the place of birth.
There is a huge lack of information for parents and the professionals who care for these newborns concerning the importance of NHS in the early detection of congenital hearing disorders and of late or progressive onset3,18.
There has been a great difficulty concerning the mothers' understanding of the importance of NHS in the early diagnosis and treatment of babies with hearing disorders. Many mothers have reported financial difficulties in commuting to the hospital for the follow up visits.
We must stress that our hospital cares for many teenage mothers, some with difficulties in coping with the situation of an unplanned pregnancy and without much interest in NHS. We also have a lot of immigrant patients whom, besides having the difficulties aforementioned, also have some worsening factors such as: communication challenges, some illegally living in the country and who do not return to the hospital in fear of being identified.
Thus, studies which have focused the causes for high rates of evasion, considering the social and demographic factors of the population considered, are needed in order to overcome this challenge.
According to the Brazilian Committee on Hearing Loss During Childhood (1999) and the
JCIH (2007), the rate of false-positives should not be higher than 3.0% in relation to the total number of children assessed. The rate of false-positives found in the present study (0.1%) is within recommended levels.
From our sample, 221 newborns (14.1%) had one or more risk indicators, and such index was similar to the ones found by other authors19.
The most frequent risks found were similar to the ones found in the literature. Risk indicators associated with ICU stay11, use of ototoxic drugs11,12,19,20, congenital infection11, mechanical ventilation11,12,19 and family history of hearing loss11,20 were the most frequent risk indicators found in the literature.
The percentage number of children who failed and were referred to diagnosis was 1.7%. Such figure is in agreement with what is recommended by the Brazilian Committee on Hearing Loss (1999) and the
JCHI (2007), which suggest that such index should not be higher than 4% of the individuals assessed.
Based on the publications compiled, we noticed that the percentage of failures can be impacted by many factors: environment noise or baby noise during the screening itself, sleepiness or awareness state of the newborn, examiner's experience with the equipment being used, time of NHS program existence, number of newborns assessed, protocol and procedure utilized, besides the demographic traits of the newborn, complications and risk indicators for hearing impairment, which will be discussed below9-11,13.
In the present study, there were no statistically significant differences in the gestational age mean value among the children who passed and those who failed the NHS.
Now, birth weight was a relevant variable. The children who failed the NHS had the lowest mean weight.
Many studies have shown associations between birth weight and NHS failure and/or hearing disorders12,21. Frequently, very low birth weight newborns, have many risk indicators. These are children born asphyxiated, who needed mechanical ventilation for a long period of time, they are given ototoxic antibiotic treatment and are subject to infection and/or meningitis22.
As far as the gender variable is concerned, we did not find significant differences, which is in agreement with some studies in the literature23.
Concerning the ears tested, failures in the newborn hearing screening were similar in both ears, which are different from what was obtained from one of the compiled studies24, which found a greater occurrence of failures in the left ear.
Comparing the frequency of failures in the newborn hearing screening program among babies born at term and pre-term, a higher rate of failures was found among pre-term newborns, a result which was similar to the ones found in the literature studied21,25.
In the present study, besides the gestational age, birth weight was also important considering NHS failures.The statistical analysis showed that the rate of failures was higher among those pre-term babies with very low birth weight. Many studies have reported this same finding21.
Failures in the newborn hearing screening program were the most prevalent in the group of children with the most risk indicators.
Numerous studies have described that the more risk indicators a person has, the higher will be the likelihood of this person developing some sort of hearing disorder25,26.
Risk indicators: cranial malformation, hyperbilirubinemia, mechanical ventilation, use of ototoxic drugs, peri-intraventricular hemorrhage, ICU stay longer than 48 hours were variables associated with NHS failure.
Craniofacial malformation has been considered a risk factor associated with hearing disorders25.
It is known that hyperbilirubinemia is toxic to the hearing apparatus and to the central nervous system, and it can cause sequelae such as hearing loss, hearing neuropathy and encephalopathy. Numerous studies have described the correlation between hyperbilirubinemia and hearing disorders20,27.
Risk indicator: mechanical ventilation, also seems to be associated with hearing disorders in the literature28.
The effect of ototoxic drugs in the hearing of highrisk newborns has been compared by many authors20,28,29.
Studies have showed the presence of peri-intraventricular hemorrhage in children with hearing disorders20 and some authors have described it as one of the indicators associated with hearing neuropathy30.
The greater need to send to the ICU those newborns who failed NHS shows that such babies have a greater risk of failing NHS, since, in general, they have other factors associated25,31.
The rate of hearing disorders, considering the 1,554 babies who completed the NHS stages and the audiologic diagnostic was 0.5%. The hearing loss rate in the present study was similar to that reported by studies carried out in populations with similar traits to the ones studied10-13,15,19,20.
There were no differences between males and females insofar as the diagnosed hearing disorders are concerned. However, in relation to gestational age and weight at birth, it has been noticed a greater percentage of very low birth weight premature babies with hearing disorders. Some studies have reported a greater rate of hearing loss in premature children21. Many studies have reported that birth weight below 1,500g is one of the most frequent indicators associating hearing disorders with children6,12,20,30.
It is worth stressing the importance, not only of doing the NHS in premature babies, but also to follow up on their hearing and language development. Premature children may present a delay in their Hearing behavior when compared to their counterparts born at term32. Children with a positive family history for hearing impairment during childhood must be considered at risk for progressive and/or late hearing loss4.
In regards of the number of risk indicators presented by the group of term newborns diagnosed with hearing loss, one child did not have any risk factor, and another one had craniofacial malformation; in the group of premature babies with birth weights higher than 1,500g, the child diagnosed with sensorineural hearing loss had only the risk of family history for HL; and in the group of premature babies with birth weight below 1,500g, besides the very low birth weight risk, all the children diagnosed with hearing disorders remained in the ICU for a period longer than 48 hours, four needed mechanical ventilation, four used ototoxic drugs and two had peri-intraventricular hemorrhage. A proper interview made by the NHS team is fundamental to identify the risks associated with hearing loss and the need to follow these children up, even if they had normal results in the NHS.
It is recommended that all children who spent over five days in the ICU be referred to hearing assessment by means of the BAEP, in order to detect children with hearing neuropathy spectrum.
Many studies have shown an association between hearing disorders and risk indicators for hearing loss6,33.
Although children with a risk for hearing loss have a greater likelihood of having disorders, there are a considerable number of children diagnosed with hearing loss who do not have any risk factors. In the present study, one of the eight children diagnosed with hearing disorders did not have any risk factor.
Compiled studies in the literature report significant rates of children diagnosed with hearing loss who did not have risk indicators for hearing disorders33.
There was a statistically significant association among risk indicators for hearing disorder: family history for hearing loss, craniofacial malformation, need for mechanical ventilation, use of ototoxic drugs, periintraventricular hemorrhage, ICU stay for over 48 hours and very low birth weight.
Based on the results found in the present study we can make many suggestions to the NHS team. Besides being a well-trained and well-structured team, it must me committed to performing NHS every day of the week, have a proper and effective follow up of these babies who failed NHS and those who had risk indicators for hearing loss and must invest in practice and experience in order to increase the efficacy of the Program10,33.
The importance and implementation of the NHS programs in Brazil has grown substantially; however, there are numerous challenges preventing it from being effective, especially in maternities which serve the low income population, since the rate of evasion among this population during the NHS program is high. Studies which analyze the best method to bring mothers awareness about the importance of the audiological diagnosis and the minimization of evasion factors in the NHS process are valid in order to make the NHS program more effective.
CONCLUSIONS1. The rate of detected failures by otoacoustic emissions and the use of the cochleo-eyelid reflex was 1.7%;
2. The prevalence of hearing disorders found in the present study was 0.5%;
3. The very low birth weight newborns have higher newborn hearing screening failure rates and hearing disorders;
4. The failure rate in newborn hearing screening was associated in a statistically significant fashion with the risk indicators: craniofacial malformation, hyperbilirubinemia, need for mechanical ventilation, use of ototoxic drugs, presence of peri-intraventricular hemorrhage, stay in middle to high risk the neonatal ICU for more and 48 hours and birth weight below 1,500g;
5. Hearing disorders were significantly associated with the variables: family history for hearing loss, craniofacial malformation, use of ototoxic drugs, need for mechanical ventilation, presence of peri-intraventricular hemorrhage, stay in middle to high risk neonatal ICU for over 48 hours and birth weight below 1,500g.
REFERENCES1. Johnson JL, Mauk GW, Takekawa KM, Simon PR, Sia CCJ, Blackwell PM. Implementing a statewide system of services for infants and toddlers with hearing disabilities. Semin Hear. 1993;14(1):105-18.
2. Sartorato EL, Guerra ATM. Genes do silêncio: a complexidade clínica da surdez genética. Rev. Bras. Otorrinolaringol. 2002;68(6):903-6.
3. Comitê Brasileiro sobre Perdas Auditivas na Infância. Recomendação 01/99. Dispõe sobre os problemas auditivos no período neonatal. Jornal do Conselho Federal de Fonoaudiologia. 2000;5:3-7.
4. Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Joint Committee on Infant Hearing. Pediatrics. 2007;120(4):898-921.
5. Lewis DR, Marone SAM, Mendes CA, Cruz OLM, Nóbrega M. Comitê multiprofissional em saúde auditiva: COMUSA. Braz J Otorhinolaryngol.2010;76(1):121-8.
6. Davis H, Silverman SR. Auditory Test Hearing Aids. In: Davis H, Silverman SR. Hearing and Deafness. New York: Holt Rinehart and Winston; 1970.
7. Canale A, Favero E, Lacilla M, Recchia E, Schindler A, Roggero N, et al. Age at diagnosis of deaf babies: a retrospective analysis highlighting the advantage of newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006;70(7):1283-9
8. Taterrsall H, Young A. Deaf children identified through newborn hearing screening: parents experiences of the diagnostic process. Child Care Health Dev. 2006;32(1):33-45.
9. Korres SG, Balatsouras DG, Nikolopoulos T, Korres GS, Ferekidis E. Making universal newborn hearing screening a success. Int J Pediatr Otorhinolaryngol. 2006;70(2):241-6.
10. Lévêque M, Schmidt P, Leroux B, Danvin JB, Langagne T, Labrousse M, et al. Universal newborn hearing screening: a 27-month experience in the French region of Champagne-Ardenne. Acta Paediatr. 2007;96(8):1150-4.
11. Azevedo RF, Paschoal CP, Azevedo MF, Santos AM, Furia CLB. Avaliação da implantação de programa de triagem auditiva neonatal em hospital de nível secundário. Rev Paul Pediatr. 2004;22(2):77-84.
12. Barreira-Nielsen, C, Futuro Neto AH, Gattaz G. Processo de implantação de Programa de Saúde Auditiva em duas maternidades públicas. Rev Soc Bras Fonoaudiol. 2007;12(2):99-105.
13. Chapchap MJ, Ribeiro FM, Leite R, Ueda S, Yoshida V. Etapas da Triagem Auditiva Neonatal e indicadores de qualidade. 2007. [Apresentado no III Encontro Nacional de Triagem Auditiva Neonatal. São Paulo, 2007]. Disponível em www.gatanu.org.
14. Downs MP, Sterritt GM. A guide to newborn and infant hearing screening programs. Arch Otolaryngol. 1967;85(1):15-22.
15. Babac S, Djeric D, Ivankovic Z. Newborn hearing screening. Srp Arh Celok Lek. 2007;135(5-6):264-8.
16. Doyle KJ, Kong YY, Strobel K, Dallaire P, Ray RM. Neonatal middle ear effusion predicts chronic otitis media with effusion. Otol Neurotol. 2004;25(3):318-22.
17. Korres S, Nikolopoulos T, Ferekidis E, Gotzamanoglou Z, Georgiou A, Balatsouras DG. Otoacoustic emissions in universal hearing screening: which day after birth should we examine the newborns? ORL J Otorhinolaryngol Relat Spec. 2003;65(4):199-201.
18. Machado MS, Oliveira TMT, Cóser PL. Triagem auditiva neonatal universal: projeto piloto no Hospital Universitário de Santa Maria (RS)-Brasil. Pró-fono. 2002;14(2):199-204.
19. Durante AS, Carvallo RMM, Costa MTZ, Ianciarullo MA, Voegels RL, Takahashi GM, et al. A implementação de programa de triagem auditiva neonatal universal em um hospital universitário brasileiro. Pediatria (São Paulo). 2004;26(2):78-84.
20. Tiensoli LO, Goulart LMHF, Resende LM, Colosimo EA. Triagem auditiva em hospital público de Belo Horizonte, Minas Gerais, Brasil: deficiência auditiva e seus fatores de risco em neonatos e lactentes. Cad. Saúde Pública. 2007;23(6):1431-41.
21. Pereira PKS, Martins A, Vieira MR, Azevedo MF. Programa de triagem auditiva neonatal: associação entre perda auditiva e fatores de risco. Pró-fono. 2007;19(3):267-78.
22. Segre CAM. Prevalência de perda auditiva em recém-nascidos de muito baixo peso. J Pediatr. 2003;79(2):103-4.
23. Lima GML, Marba STM, Santos MFC. Avaliação auditiva em recémnascidos internados em unidades de terapia intensiva e de cuidados intermediários: triagem e acompanhamento ambulatorial. Rev Ciências Médicas. 2005;14(2):147-56.
24. Simonek MCS. Ocorrência de respostas falso-positivas em um programa de triagem auditiva neonatal universal: possíveis causas e soluções. [tese]. São Paulo: Universidade Federal de São Paulo; 2006.
25. Korres S, Balatsouras DG, Vlachou S, Kastanioudakis IG, Ziavra NV, Ferekidis E. Overcoming difficulties in implementing a universal newborn hearing screening program. Turk J Pediatr.2005;47(3):203-12.
26. Andrade AN, Amador HC, Gil D, Azevedo MF. Indicadores de alteração auditiva central em uma população ambulatorial. Fono Atual. 2005;34(8):25-33.
27. Silva DPC, Martins RHG. Analysis of transient otoacoustic emissions and brainstem evoked auditory potentials in neonates with hyperbilirubinemia. Braz J Otorhinolaryngol. 2009;75(3):381-6.
28. Weichbold V; Nekahm-Heis D; Welzl-Mueller K. Universal newborn hearing screening and postnatal hearing loss. Pediatrics.
2006;117(4):631-6.
29. Câmara MFS. Efeito de fármacos ototóxicos na audição de recémnascidos de alto risco. [tese]. São Paulo: Universidade Federal de São Paulo; 2005.
30. Berg AL, Spitzer JB, Towers HM, Bartosiewicz C, Diamond BE. Newborn hearing screening in the NICU: profile of failed auditory brainstem response/passed otoacoustic emission. Pediatrics. 2005;116(4):933-8.
31. Jardim I. Emissões otoacústicas evocadas por estímulos transientes e potencial evocado auditivo de tronco encefálico automático na triagem auditiva neonatal. [tese]. São Paulo: Universidade de São Paulo; 2006.
32. Azevedo MF. Avaliação e acompanhamento audiológico de neonatos de risco. Acta AWHO. 1991;10(3):107-16.
33. Pastorino G, Sergi P, Mastrangelo M, Ravazzani P, Tognola G, Parazzini M, et al. The Milan Project: a newborn hearing screening programme. Acta Paediatr. 2005;94(4):458-63.
1. MSc, Speech and Hearing Therapist.
2. PhD; Professor at the Department of Speech and Hearing Therapy.
3. PhD; Professor at the Department of Pediatrics.
Send correspondence to:
Raquel Mari Onoda
Rua dos Lírios, 195, apto. 82, Mirandópolis
São Paulo - SP. CEP: 04047-040
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on September 07, 2010
Accepted on May 01, 2011. cod. 7309
National Council for Scientific and Technological Development - CNPq


