INTRODUCTIONStridor is a symptom and not a diagnosis and it is the most predominant characteristic of airway obstruction in children7. It is generated by the turbulence of air during the passage through a partially obstructed site. Stridor may be inspiratory, expiratory and biphasic, depending on location and type of respiratory tree abnormalities. In infants, the main causes are laryngeal anomalies. Among them, we may cite laryngomalacia and subglottic stenosis 4.
The term laryngomalacia was introduced in 194210 and described the collapse of supraglottic structures during inspiration. Before that, congenital laryngeal diseases that generated stridor were described all together as congenital laryngeal stridor 14. Despite the fact that laryngomalacia is the most common underlying disease in most newborns and infants who have stridor 11, we should not ignore the other causes8. In 5 to 37% of the cases we can identify secondary damage to the airways, which are only diagnosed by fibronasolaryngobronchoscopy 1.
Subglottic stenosis may be congenital or acquired. The congenital pathology is related to failure in recannulation of the laryngeal lumen during embryonic development4. The most common acquired form is normally related to endotracheal intubation, but there are also secondary cases to caustic ingestion, surgical complications, granulomatous diseases and tumors, among others. It is not easy to differentiate an acquired form from a congenital one, because sometimes we do not have a chance to analyze stridor before airway handling by other professionals because of the need to control acute respiratory function, such as in cases of endotracheal intubation, cricothyroidectomy and tracheostomy.
Even though laryngomalacia and subglottic stenosis are the most frequent causes of stridor in infants, there are other airway affections that cause stridor and should not be treated based on inferences or assumptions7. The purpose of the present study was to describe the main causes and comorbidity associated to stridor in children seen in our hospital.
MATERIAL A ND METHODWe conducted a transversal study of prospective planning, from March 2000 to April 2001 involving 125 children. We included in the protocol all patients referred to the Service of Pediatric Otorhinolaryngology at Hospital da Criança Santo Antônio (HCSA), Complexo Hospitalar Santa Casa de Porto Alegre (CHSCPA) because of stridor. Nearly half of the patients (48%) came from the Intensive Care Unit of HCSA. Out of 125 subjects, 69 (55.2%) were male and 56 (44.8%) were females subjects. The mean age at presentation was 19 months, ranging from 4 days to 14 years.
Patients were assessed according to the protocol previously established by the authors and approved by the Ethics Committee of CHSCPA, including questions about age, gender, current and previous clinical history, physical examination and associated comorbidity, besides standardized fibronasolaryngobronchoscopy. The test included assessment of nasal fossae, rhinopharynx, oropharynx, hypopharynx, larynx, trachea, and main bronchia on the right and left. The endoscopic test was performed by the senior author of the present study. The protocol was filled out by the same professional, creating a database inputted into Excel (Microsoftâ) software. The analyses were all descriptive in percentage.
We planned a secondary analysis of the two most common causes of stridor in the largest world series: laryngomalacia and subglottic stenosis. Laryngomalacia was classified into 5 types - Type I: prolapse of enlarged cuneiform cartilages; Type II: exaggerated omega format of epiglottis cartilage, which is long and tubular and bends over itself during inspiration; Type III: anterior and medial collapse of arytenoids during inspiration; Type IV: posterior projection of epiglottis during inspiration, and Type V: short aryepiglottic fold. Subglottic stenosis was also divided according to obstruction potential in 4 types - type I (25% of obstruction); type II (between 51 and 70%); type III (between 71 and 90%) and type IV (>90%).
RESULTSIn the clinical assessment, the main comorbidity manifestations found were: prolonged intubation in 36 cases (28.8%), gastroesophageal reflux disease in 23 (18.4%) and congenital malformations in 19 (15.2%) (Graph I), being that 13 children were premature (10.4%).
Among the congenital malformations, the main ones were cardiac malformations in 9 cases (7.2%) and neural tube malformations in 3 cases (2.4%) (Graph II).
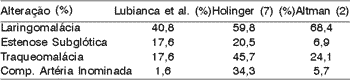
Table I. Main Laryngeal and Tracheal Anomalies that Cause Stridor.

Table II. Main Laryngeal Anomalies that Cause Stridor (< 30 months).
Considering the airway regions, the larynx concentrated the highest number of abnormalities (Table I). Laryngomalacia was present in 51 patients (40.8%), subglottic stenosis in 22 (17.6%), vocal fold edema in 12 (9.6%), vocal fold granuloma in 5 patients (4%), vocal fold paralysis in 8 (6.4%) and anterior laryngeal web in 1 case (0.8%). Table II compares the data collected here to data reported by Holinger1 in infants younger than 30 months. Except for the difference in size of the two samples, percentages are very similar (Table II).
In our series of cases, there were 15 (29.4%) cases of type I laryngomalacia, 4 (7.8%) of type II, 4 (7.8%) of type III, 11 (21.5%) of type IV and 11 (21.5%) of type V. Six cases were not classified (11.7%). Approximately 32% of the patients presented subglottic stenosis type I, 45 % type II, 9 % type III and 14 % type IV. The tracheal examination was normal in 89 cases (71.2%). In 33 cases (26.4%) the trachea was abnormal and it was not assessed in 3 other cases. The main tracheal alteration detected was tracheomalacia, present in 22 patients (17.6%). The examination of carina and main bronchia was normal in 115 cases and it was not performed in 10 cases.
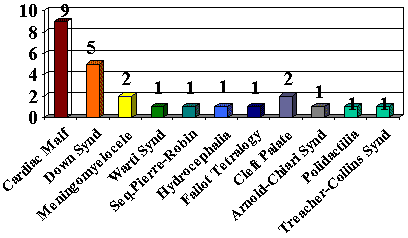
Graph I. Comorbidity Associated to Stridor.
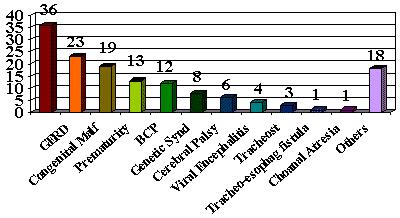
Graph II. Congenital Malformations and Genetic Syndromes Associated to Stridor.
Airway congenital anomalies represent the main cause of stridor in newborns and infants2, 7, 8. However, most of the children may not manifest stridor since birth. If present at birth, the causes are normally fixed obstruction, such as laryngeal webs and congenital subglottic stenosis. Stridor is frequently manifested gradually or as exacerbation periods (feeding, crying, position), leading to dyspnea, cyanosis or apnea12. In our series of cases, the presentation age ranged from 4 days to 14 years (the oldest one was a patient with cerebral palsy and tracheostomy, with obstructive peritracheal granulation), indicating how wide the age range is for the identification of symptoms, although the mean age was 19 months of age. There was a slight predominance of male subjects. Similar data were found in series of patients with laryngomalacia 9 and sleep apneas5. However, the male predominance in cases of congenital tracheal anomalies and congenital and acquired tracheal stenosis may suggest that the male population has a higher risk of having airway obstructive damage7.
The assessment of patients with stridor requires complete detailing of symptoms, such as onset date, characteristics, intensity, aggravating and relieving factors and complications. Inspiration stridor is characteristic of damage above the thoracic cage. It is generated by the collapse of laryngeal structures caused by the negative pressure created by the thoracic cage by the inspiration movements. The subglottic stenosis damage, even if extra-thoracic, produces biphasic stridor because it does not have morphology modified by airway pressure. Tracheomalacia mainly produces expiratory stridor, such as asthma. The exception is the case of intra and extra-thoracic tracheal compromise, leading to biphasic stridor.
However, simple characterization of symptoms is not enough to come to a precise diagnosis. Holinger 7 reported a series of 219 patients in which 58 cases had the wrong diagnosis corrected by fibronasolaryngobronchoscopy. The main wrong diagnoses were asthma, whooping cough and bronchiolitis. That is the reason why the endoscopic examination is essential, since it defines the exact cause of the symptom, in addition to excluding other airway affections. In another study2, 37% of the patients had more than one airway lesion. No other test, such as fluoroscopy, barium esophagus studies or lateral neck x-rays, is as define and clarifying for airway damage15 as endoscopy.
The endoscopy revealed abnormalities in almost all cases. The main alterations were laryngeal ones, and there was a predominance of laryngomalacia and subglottic stenosis. Vocal folds were normal in 86 of the cases (68.8%). The test revealed that 22 cases had omega epiglottis. Omega epiglottis alone does not justify stridor 3, but it may be a contributing factor to the onset of symptoms, when associated to other alterations8. Tracheomalacia was the main alteration found in the trachea, which is in agreement with other studies.
Laryngomalacia was the most commonly found congenital laryngeal anomaly. It is normally a self-limited condition that may produce severe episodes of apnea, cor pulmonale and developmental delays17. Its origin is correlated to laryngeal neurological immaturity. Aryepiglottic folds may be short, omega epiglottis and cuneiform cartilages may be extremely big. These are conditions that favor laryngeal prolapse during inspiration. Laryngomalacia symptomatology is characterized by stridor that starts in the first two weeks of life, present at rest and/or activity. Stridor is also related to the child's position, usually aggravated in the supine position and relived in the prone position. The respiratory compromise of laryngomalacia is not always as severe and the child does not present cyanosis and dyspnea. Feeding difficulties, in turn, are normally noticed. The clinical evolution is benign in most patients, and resolution of symptoms takes place up to 18 months of age4. However, in some cases, there may be respiratory complications that require supraglottoplasty 13, 17.
Subglottic stenosis (SS) was the second most frequent lesion in our cases. SS is defined as a lumen measure of the cricoid cartilage below 4mm in term babies2. Incomplete failures of laryngeal recannulation during embryonic life determine the types of SS. Symptomatology may not manifest up to the occurrence of a triggering event. Most of the cases are present after respiratory tract infections, but they may also manifest after prolonged periods of intubation. Gastroesophageal reflux disease (GERD) is also associated to the development of SS16. Halstead 6 has recently conducted a study showing that GERD has a causal role in the development of SS. The same author noticed that clinical treatment with anti-reflux drugs might solve symptomatology in one third of the cases. The treatment of SS may be plain observation in less severe cases, because of remission of symptoms as a result of growth. However, some patients require surgical treatment, such as balloon dilation or specific dilation methods, use of carbon dioxide laser and laryngotracheal reconstruction, which are the main surgical options.
Congenital or acquired laryngeal anomalies are commonly followed by comorbidity. Prolonged intubation and congenital malformations were good examples in the studied cases. Among the malformations, cardiac ones were the most representative in the sample, in agreement with the data from other authors2. Infections of the upper respiratory tract are important comorbidity because they trigger the symptomatology in many cases4. However, most of the cases are treated by pediatricians in the emergency room and ICU of our hospital and they do not require our management. Finally, prematurity may be involved in the genesis of some causes of stridor, since it is a determining factor for neurological immaturity of the respiratory tree2, and it was seen in many of the analyzed patients.
Some potential limitations to our study should be mentioned. Since the study had a diagnostic focus, we did not report in details the therapeutic measures adopted in the cases and decided not to discuss them. As to our sample, our children represented a very different population when compared to regular outpatient populations in which the Otorhinolaryngologist occasionally finds one case of stridor. Most of our patients were referred from the ICU of the hospital, which represented extreme cases of stridor. Nevertheless, the causes of stridor did not vary significantly compared to other studies (see Tables I and II). The fact that we analyzed a highly selected sample explains the absence of infectious and cardiac causes of stridor, among others, which are common in samples that represent regular outpatient populations.
CONCLUSIONEven though we have assessed patients that were more selected that most of the subjects included in world studies, laryngeal anomalies, either congenital or not (laryngomalacia and subglottic stenosis) were also the main causes of stridor in our sample. In some cases that had history of prolonged intubation, it was difficult to differentiate congenital from acquired subglottic stenosis. The presence of comorbidity was frequent and it is one of the factors that may help the selection of patients that need endoscopic analysis.
REFERENCES1. Albert D, Leighton S. Stridor and Airway Management. In: Cummings CW, Fredrickson JM, Harker LA, Krause CJ, Schuller DE, Richardson MA. Pediatric Otolaryngology Head & Neck Surgery, 3rd ed., St. Louis, Mosby, 1998. p.285-302.
2. Altman KW, Wetmore RF, Marsh RR. Congenital Airway Abnormalities in Patients Requiring Hospitalization. Arch Otolaryngol Head Neck Surg 1999;125:525-528.
3. Belmont JR, Grundfast K. Congenital Laryngeal Stridor (Laryngomalacia): Etiologic Factors and Associated Disorders, Ann Otol Rhinol Laryngol 1984;93:430-437.
4. Cotton RT, Prescott CAJ. Congenital Anomalies of the Larynx. In: Practical Pediatric Otolaryngology, 1st ed., Philadelphia: Lippincott-Raven Publishers; 1999.
5. Guilleminault C, van den Hoed J, Mitler MM. Clinical overview of the sleep apneas syndromes. In: Sleep Apnea Syndromes. New York: Allan R. List Inc; 1978.
6. Halstead LA. Gastroesophageal reflux: A critical factor in pediatric subglottic stenosis. Otolaryngol Head Neck Surg 1999;120:683-688.
7. Holinger LD. Etiology of Stridor in the Neonate, Infant and Child. Ann Otol Rhinol Laryngol 1980;89:397-400.
8. Holinger LD, Konior RJ. Surgical Management of Severe Laryngomalacia. Laryngoscope 1989;99:136-142.
9. Holinger PH, Johnston KC, Schiller F. Congenital Anomalies of the Larynx. Ann Otol Rhinol Laryngol 1954;63:581-606.
10. Jackson C, Jackson CL. Diseases and Injuries of the Larynx. First edition. New York: Macmillan, 1942.
11. Mancuso RF, Choi SS, Zalzal ZH, Grundfast KM. Laryngomalacia. Arch Otolaryngol Head Neck Surg 1996;122:302-306.
12. Richardson MA, Cotton RT. Anatomic abnormalities of the pediatric airway. Ped Clin of North Am 1984;31:821-834.
13. Roger G, Denoyelle F, Triglia JM, Garabedian EM. Severe Laryngomalacia: Surgical indications and results in 115 patients. Laryngoscope 1995;105:1111-1117.
14. Sutherland GA, Lock HL. Congenital Laryngeal Obstruction. Lancet 1897;2:653-655.
15. Tunkel DE, Zalzal GH. Stridor in infants and children: ambulatory evaluation and operative diagnosis. Clin Pediatr (Phila.) 1992;31:38-55.
16. Yellon RF. The Spectrum of Reflux-Associated Otolaryngologic Problems in Infants and Children. Am J Med 1997;103(5A): 125-129.
17. Zalzal GH, Anon JB, Cotton RT. Ann Otol Rhinol Laryngol 1987;96:72-76.
[1] Joint Professor, Discipline of Otorhinolaryngology, Federal College Foundation of Medical Sciences, (FFCMPA) and Coordinator of the Service of Pediatric Otorhinolaryngology, Hospital da Criança Santo Antônio (HCSA), Complexo Hospitalar Santa Casa de Porto Alegre (CHSCPA).
[2] Faculty Professor, Discipline of Pediatrics, FFFCMPA.
[3] Resident Physicians, Service of Otorhinolaryngology, CHSCPA.
[4] Undergraduates, FFFCMPA.
Study conducted at the Service of Pediatric Otorhinolaryngology, HCSA, CHSCPA and Discipline of Otorhinolaryngology, FFFCMPA.
Address correspondence to: José Faibes Lubianca Neto - Rua Mostardeiro 157, sala 608 - 90430-001 - Porto Alegre RS - Brazil - E-mail: jlubianca@terra.com.br
Study presented at II Congresso Triológico de Otorrinolaringologia, in Goiânia, 2001.
The project was financially supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and by CNPq PIBIC/2000.


