INTRODUCTIONCrille described radical neck dissection in 1906 as a procedure for en bloc removal of the sternocleidomastoid muscle, the internal jugular vein, the accessory nerve, and lymphatic and fatty tissues. The purpose of this procedure was to assure that most of the lymph nodes were removed, thereby maximizing its therapeutic effect and improving staging.1 Martin later defended this idea by arguing in favor of removing the abovementioned three non-lymphatic structures as a method for achieving full neck lymphadenectomy.2
Although full lymphadenectomy is achievable, classic radical neck dissection causes significant sequelae due to the removal of non-lymphatic structures. Thus, 40 years ago, efforts were made to preserve these structures - in particular the accessory nerve - when not affected by the tumor; these procedures became known as modified radical neck dissections.3 This trend continued, and methods were found to preserve the internal jugular vein and the sternocleidomastoid muscle, which resulted in the so called functional neck dissection.4,5
Notwithstanding the increasing popularity of these modified radical neck dissection procedures, a major issue is the impact of these modifications on surgical efficacy. We therefore investigated the number of recovered lymph nodes in each technique and the impact on regional recurrence and survival to test this effect.
MATERIAL AND METHODThis retrospective study investigated patients who had undergone neck dissection with the removal of lymphatic levels I to V, with or without removal of the accessory nerve, the internal jugular vein, or the sternocleidomastoid muscle. Patients with tumors of the lower mouth and oropharynx diagnosed histologically as squamous cell carcinoma were included. Patients who underwent extended neck dissections, preoperative radiotherapy or chemotherapy, or prior surgery were not included. For the statistical analysis patients were grouped according to the number of structures preserved into three groups: radical neck dissection (RND), neck dissection preserving the accessory nerve (MRND + XI), and neck dissection preserving the accessory nerve and the internal jugular vein (MRND IJV+XI). The AJCC 20026 stating method was applied to all patients.
The Stata 10.1 (StataCorp, Texas, USA) software for MacOS X was used for the statistical analysis. Values are presented as the mean and standard deviation (SD). The analysis of variance (ANOVA) with Scheffé's test for multiple comparisons among groups was applied. The Kaplan-Meier and Cox methods were applied for assessing survival and regional recurrences; the hazard ratio (HR)value was indicated, and the confidence interval (CI) was 95%. Significant p values were p<0.05.
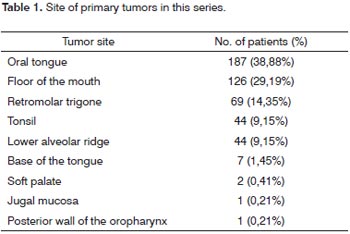
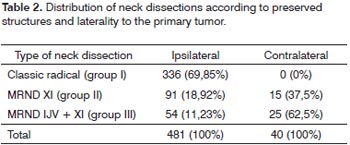
RESULTSThere were 481 patients that fit the inclusion criteria. There were 424 (88.15%) males and 57 (11.85%) females. The mean age at the time of treatment was 55.97 years (SD 10.0 years). The most common primary site was the oral tongue (187 cases; 38.88%), followed by the floor of the mouth (126 cases; 29.19%), the retromolar trigone (69 cases; 14.35%), and the tonsils and lower gingival margin (44 cases; 9.15%). Table 1 shows the complete list of primary sites. Staging of the primary tumor revealed 19 T1 patients (3.95%), 202 T2 patients (42.0%), 148 T3 patients (30.77%), and 112 T4a patients (23.28%). There were 214 N0 patients (44.49%), 79 N1 patients (16.42%), 5 N2a patients (1.04%), 138 N2b patients (28.69%), 39 N2c patients (8.11%), and 6 N3 patients (1.25%). Bilateral radical neck dissection was carried out in 40 patients, resulting in a total 521 radical neck dissections. Contralateral dissection to the primary tumor was selective in 85 cases, and will not be analyzed in this study. Table 2 shows the distribution of neck dissections according to each group and laterality.
The mean number of dissected lymph nodes was 44.92 (SD - 16.45) in RND; 44.16 (SD - 15.76) in MRND +XI; and 56.02 (SD - 22.91) in MRND IJV+XI. ANOVA showed a statistically significant difference among groups (p<0.001),which was further investigated with the Scheffé test (Table 3); this test revealed that significantly more lymph nodes were recovered in patients undergoing modified neck dissections.

Follow-up ranged from 0.3 to 322.23 months (mean - 60.79 months, SD - 22.39 months). Local recurrence occurred in 122 cases; 74 of these were neck recurrences, and 46 were distance recurrences; 250 patients had recurrences in at least one site. Statistically significant factors for neck recurrences were age (p=0.037), staging of the neck (p=0.006), primary tumor thickness (p=0.002), and postoperative radiotherapy (p=0.046). In this model the type of neck dissection had no impact on regional recurrence(p=0.878, Table 4). Figure 1 shows the neck recurrence curves according to the type of neck dissection. Harrell's agreement test revealed a higher predictive ability of themodel without including the type of neck dissection (Table 5). Statistically significant factors for disease-free survival were the T stage (p=0.001), the N stage (p=0.001), the presence of lymphatic vascular embolization (p=0.019), and tumor thickness (p=0.008). The type of neck dissection had no significant impact (p=0.185, Table 6). Survival curves on Figure 2 show a similar behavior in both groups. Harrell's agreement test showed that the model without the type of neck dissection was superior to the model with the type of neck dissection (Table 7).
DISCUSSIONThe extent of neck dissection has been an important topic for debates. Based on surgical and autopsy data, removal of lymphatic and fatty tissues without removing non-lymphatic structures was found to be oncologically sound. Neck lymph nodes are located within fibro-adipose tissue next to nerves and blood vessels; there are no lymph nodes in the muscle aponeurotic fascia.4 Bocca and Pignataro have confirmed these findings.5
Resection of the accessory nerve has been routinely avoided in selected cases since the 1950s because of the associated morbidity. It is preserved whenever there is a clear cleavage plan between the nerve and compromised lymph nodes.7
A criticism of modified radical neck dissections is the decrease in recovered lymph nodes relative to the number of preserved non-lymphatic structures. According to Busaba et al., classical radical neck dissection had a significantly higher number of recovered lymph nodes compared to the variations of this procedure.8 These authors suggested that this could have a negative impact on the prognosis of patients. However, Siddiquee et al. contested this finding by showing that classical and modified radical neck dissection had comparable oncologicalefficacy.9 This confirmed previously published papers that had also demonstrated a similar oncological efficacy of modified neck dissection, which has lower morbidity, especially in shoulder function.10
CONCLUSIONOur series showed that the modified radical neck dissection has a similar oncological efficacy to the classical radical neck dissection, and that contrary to previous arguments in the literature, the number of recovered lymph nodes is higher than that in classical neck dissection. There is thus no loss in staging on pathology and the prognosis of patients, or in their survival and disease-free interval.
REFERENCES1. Crile G Sr. Excision of the cancer of the head and neck with reference to the plan of dissection based on 132 patients. JAMA. 1906;47:1780-90.
2. Martin HE, Del Valle B, Ehrlich, Cahan WG. Neck dissection. Cancer. 1951;4:441-99.
3. Medina JE, Rigual NR. Neck dissection. In: Bailey BJ (Ed). Head and Neck Surgery - Otolaryngology, vol 2. Philadelphia: JB Lippincott, Co.; 1192-220.
4. Suarez O. El problema se lãs metastasis linfaticas y alejadas del cancer de laringe e hipofaringe. Rev Otorhinolaryngol. 1963;23:83-9.
5. Bocca E, Pignataro O. A conservation technique in radical neck dissection. Ann Otol Rhinol Laryngol. 1967;76:975-87.
6. Greene FL, Compton CC, Fritz AG, Shah JP, Winchester D. PAJCC Cancer Staging Atlas. Springer Chicago; 2006, Il. P.11-76.
7. Branderberg JH, Lee CY. The XI nerve in radical neck surgery. Laryngoscope. 1981;91:1851-9.
8. Busaba NY, Fabian RL. Extent of lymphadenectomy achieved by various modifications of neck dissection: a pathologic analysis. Laryngoscope. 1998;109:212-5.
9. Siddiquee BH, Amin SA, Sharif A. Comparative study of radical VS modified radical neck dissection in metastatic neck gland. Mymesingh Med J. 2007;16(1):25-8.
10. Buckley JG, Feber T. Surgical treatment of cervical node metastases from squamous cell carcinoma of the upper aerodigestive tract: evaluation for modifications of neck dissection. Head Neck. 2001;23(10):907-15.
1. Physician.
2. Doctorate, medical pathologist, Pathology Department, A. C. Camargo Hospital.
3. Livre-Docente in onchology. Director of the Head & Neck and Otorhinolaringology Department, A. C. Camargo Hospital.
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on July 29, 2009;
and accepted on September 9, 2009. cod. 6532


