INTRODUCTIONHearing loss in Brazil has been diagnosed on average from 2 to 3 years of age1. Up to diagnosis, the child misses auditory information and interrupts the communication process. Audiologists, otorhinolaryngologists and pediatricians are concerned about early diagnosis and intervention, fitting of hearing aids and family education in order to minimize negative effects.
According to WHO data, 40% of the pregnant women experience some complications and 15% of them need specific care to reduce risk of life for the mother and the child. Complications may also be sudden and unpredictable. In Brazil, Nobrega2 found 36.5% of children with hearing loss whose mothers had some kind of complication during pregnancy.
The prevalence of children with congenital, profound, bilateral and sensorineural hearing loss equal or greater to 80dBHL is 1:1,000 live births and for children with risk indicators of moderate, severe and profound hearing loss it grows to 6:1,0003. The incidence found by White et al4 was 3:1,000 children in the normal nursery and 23:1,000 in the neonatal ICU.
Brazil is a developing country, with large territorial extension and significant social-economic and cultural differences, which presents a birth rate of 3 million children a year, according to data published by SUS (1999), and where 53% of the population has a monthly income of about two minimum salaries5.
These alarming facts make us adapt techniques and use equipment whose costs are affordable by more deprived regions that do not have financial resources or specialized professionals.
In addition, in Brazil, pre-natal examinations are not always properly made, if made, a fact that may have serious implications on the baby because of lack of education and information.
The present prospective study aimed at testing a methodological sequence and its results for the audiologic assessment of the newborn, with and without risk factors for hearing loss, children of mothers with and without gestational risk.
MATERIAL AND METHODWe assessed 174 newborns of both genders, born at the Maternity of Hospital das Clínicas, Medical School, University of São Paulo (BAM-HC, FMUSP), and their mothers.
The criteria for inclusion of the newborns were the following:
a) Control group (GA): term babies, clinically normal, with no risk indicators for hearing loss, children of healthy mothers without neurological history.
b) Experimental group: newborns with adjusted gestational age equal or greater than 37 weeks, up to 28 days of life adjusted on the assessment day and about to receive hospital discharge, born to gestational risk mothers. The group was divided into babies without risk indicators for hearing loss, group GB and babies with risk indicators for hearing loss, group GBI.
The criteria for hearing loss risk indicators were based on the recommendations of the Joint Committee On Infant Hearing, 19946, plus other four factors (marked with *). They are: family history of hereditary hearing impairment; intrauterine infections; cranial-facial anomalies, including pinna and external auditory canal; weight at birth below 1,500g; hyperbilirrubinemia for levels greater than for blood transfusion; ototoxic drug including, but not limited to, aminoglycoside used for more than 5 days or associated with diuretics; bacterial meningitis; Apgar between 0-4 in the first minute and 0-6 in the fifth minute, indicating perinatal asphyxia; mechanical ventilation for 5 days or more; signs or other findings associated with known syndromes that include sensorineural and/or conductive hearing loss; * permanence in incubator for more than 48 hours; * parents' consanguinity; * parents' positive HIV; * parents' alcohol/drug abuse.
We used the otoacoustic emissions device DP2000 - Distortion Product Otoacoustic Emission Measurement System STARKEY -, with its original accessories, installed in a laptop. We also used a series of Brazilian musical instruments: rattle, reco-reco-, cabuletê, ganzá, black-black, agogô (percussion with two small bells) and cymbals. The immitanciometer was Kamplex AZ 26 and Amplaid MK12 for auditory evoked brainstem potentials (PEATE).
DPOAE (distortion product otoacoustic emissions) were tested in frequencies f2: 984 Hz, 2.016 Hz, 3.000 Hz, 3.984 Hz and 6.000 Hz, with 1.2 frequency ratio f2/f1 and stimuli intensity of 65 and 55dB SPL, respectively.
Parents signed an Informed Consent Term to authorize the conduction of the test and the inclusion of their children data in the study, as determined and approved by the Ethics Committee on Research - CAPPesq, with the Clinical Director of HC and FMUSP.
Newborns were assessed in a quiet room next to the nursery, first with DPOAE in both ears and next, stimulated by the musical instruments.
The consciousness state of babies varied from profound sleep to awaken with minimal motor activity.
The pass/fail criteria for DPOAE included 5 parameters: similar calibration for frequencies higher than 1.500Hz; presence of OAE in at least 3 frequencies as of f2 =2.016 Hz; minimum amplitude of -10 dB; correlation between Distortion product and noise floor (PD/RF) equal or greater than 3dB for each frequency and level of intensity f1 between 60 and 70 dB SPL and f2 between 50 and 60 dB SPL.
Instrumental sound stimuli were presented at 20cm from each auricular pinna for 2 seconds with 30-second intervals between the sounds.
The criterion pass/fail for auditory behavior was: presence of one or more activities, be them reflex (cochleo-palpebral and startle reflexes); body responses (upper and/or lower limb movements); facial responses (open eyes, palpebral constriction, ocular movement, frowning, crying, making faces), or changes in consciousness state after stimulation with at least two of the following: bells, cabulete, ganza and reco-reco and presence of reflex activity for the percussion instrument and the cymbals.
PEATE was conducted by the Otorhinolaryngologist of the Department of Hearing Electrophysiology, Discipline of Otorhinolaryngology, HC, FMUSP, following the standardized protocol of the service.
Mothers of all babies who presented hearing loss risk indicators or failed in the second assessment were instructed to follow up the hearing function of their children up to the age of 2 years. These results are not part of the present study.
Figure 1 shows the study design.
Statistical Method
Data collected were compiled, analyzed and later submitted to statistical analysis. We used Chi-square Fisher test and Variance Test (ANOVA).
RESULTSAll newborns passed in the auditory behavior assessment. Therefore, the second and third stages of the assessment were performed in babies that had failed DPOAE.
We excluded 15 subjects because of lack of specific data concerning DPOAE. Thus, the total number of patients was 159 newborns submitted to history taking, amplitude analysis and PD/RF ratio of DPOAE and behavioral assessment.
We did not detect statistically significant differences between genders and ear in the three groups and, thus, analyses were made with grouped factors.
The gestational risk factors that had the highest incidences were: hypertension, diabetes, cardiopathy and maternal age (Graph 1).
GA: We evaluated 35 newborns, 20 male and 15 female.
Newborns were aged 1 to 27 days, mean age of 2.5 days of life.
GB: We evaluated 59 newborns without hearing loss risk indicators, 34 female and 25 male subjects. Gestational age ranged from 33 to 41 weeks, mean of 384-7 weeks.
GBI: We assessed 65 newborns, 30 female and 35 male subjects. Gestational age ranged from 30 to 41 weeks, mean of 364-7 weeks.
Risk indicators for hearing loss are listed in Graph 2.
Pass/Fail criteria
There was no statistically significant difference concerning incidence of failure between the groups, but we did observe a higher proportion of failures in the groups of babies born to high-risk mothers.
We considered the total of 174 subjects for the analysis, being that 41 (23.5%) failed in the first stage (Table 1).
Six out of 8 newborns failed in the first stage and presented complaints, reported by the mother, of otalgia, wheezing or tympanometric abnormality, and one of them was submitted to PEATE and showed results suggestive of conductive hearing loss. The other two did not have complaints and no tympanometric alterations were detected. However, one of them was submitted to PEATE and the results were suggestive of conductive loss.
There was statistically significant difference in mean amplitudes of DP. The frequencies of 984, 2.016 and 6.000 Hz maintained the same mean amplitude, higher than frequencies 3.000 and 3.940 Hz (Graph 3). Frequencies of 2.016, 3.984 and 6.000 Hz had the same PD/RF ratio, higher than 980 and 3.000 Hz, and there was statistically significant difference between the frequencies for all groups (Graph 4).
There was statistically significant difference of failure for frequencies and PD/RF ratio and frequencies of DPOAE. The frequency 984Hz presented the highest number of failed babies among all others of the three groups.
Reflex activities were the predominant response for sound stimulation with musical instruments.
There was no statistically significant difference when correlating type of behavioral reaction for each instrument and mean amplitude of DPOAE. There was no correlation between presence of post-instrument behavioral reaction and presence of DPOAE.
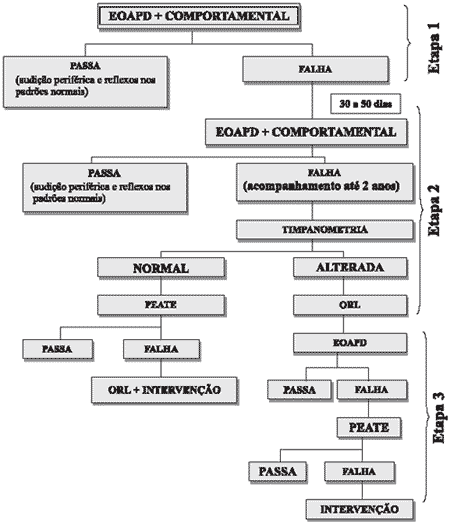
Figure 1. Study design.
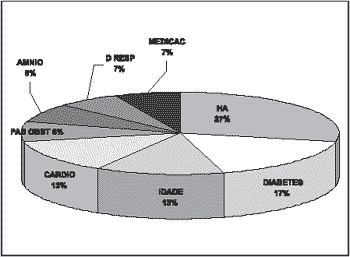
Figure 2. Gestational risk factor in groups GB and GBI.
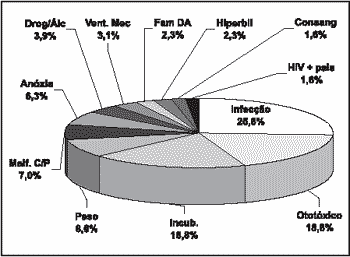
Figure 3. Risk indicators for group GBI.
Early diagnosis enables appropriate family education concerning communication of the child, leading to minimization or exclusion of the damages caused by lack of auditory information, in a stage in which the child may develop all his senses3.
In order to conduct a complete audiological assessment, it is necessary that the hearing system be checked, from the external auditory canal to the pathways and encephalic auditory centers. To that end, it is recommendable and expected to use different tests.
Otoacoustic emissions are largely used as screening tool and also as part of the newborn audiological assessment battery, as stated by different authors7, 8, 9, 10, 11.
The choice of methods for auditory tests in the present study was based on the investigation of cochlear integrity and observation of newborn reflex responses after instrument sound stimulation, assessing central auditory pathways as well.
We do not have official estimates in Brazil about the number of hearing impaired patients. Helander 12 estimated that hearing loss corresponds to 0.75% of the population of any country during years of peace, or in other words, it is estimated that approximately 1,271,583 Brazilians are hearing impaired, considering the data provided by the 2002 census. This estimate is not compatible with the estimate provided by the National Council on Speech and Language Therapy and Audiology, which recorded 2,459,214 hearing impaired people13. This difference may be explained by the fact that Brazil is a developing country and we already have epidemics of meningitis, a disease already eradicated in developed countries.
It is mandatory to follow up children who have hearing loss risk because of the likelihood of late or progressive hearing loss14, 15. The use of ototoxic drugs in nurseries is frequent and it may cause late hearing loss, together with consanguinity, genetic affections and infectious diseases. The same applies to newborns that remain in the ICU, high-risk newborns or pre-term babies that are more fragile and may be affected by diseases, procedures or drugs after discharge from nursery.
Auditory assessments are valid as from birth because myelinization of auditory nerve fibers starts on the 24th gestational week, Corti's organ is morphologically similar to the adult's on the 26th week, cochlear auditory potentials are present as of the 24th week and heart beat modifications in the fetus may be detected as a result of sound stimulation as of the 20th week16.
Logan17 warned that a screening program should avoid false-positive and false-negative results, which are harmful for the identification of the real patients. It is also important to have availability of treatment centers for patients diagnosed during the screening, so that the family does not have to be anxious and upset. The author also stated that the professionals are responsible for finding the best detection and intervention tools, providing early diagnosis also for children born away from the large centers.
The four hearing loss risk factors not proposed by the JCIH were included because many authors have pointed them as being the main causes of hearing losses1, 2, 14, 18.
The highest prevalence in maternal risk is arterial hypertension. In groups GB and GBI there were mothers aged over 35 years, an age range in which the women are more prone to experience arterial hypertension.
We found in group GBI higher prevalence of infection and use of drugs than in group GB. Toxoplasmosis, a significant risk indicator of hearing loss, was very prevalent in our sample (20.3%), increasing the number of material infections in group GBI.
The most frequent risk indicators for hearing loss in the sample were: perinatal infection (25.8%), use of ototoxic drug (18.8%), permanence in incubator (18.8%), low birth weight (8.6%) and head and neck malformation (7%). The results collected were similar to the findings of other authors1, 19.
We found a higher number of fail cases in the group of patients born to high risk mothers (GB and GBI), but there was no statistically significant difference between the groups.
We can explain the high number of fail cases in the first stage, on DPOAE, by two factors: the group of premature newborns, part of GBI, are more vulnerable to middle ear affections, cochlear maturation delay and myelinization of auditory pathways10, 18, 20, 21, 22, 23. Even when we avoid assessing the baby before the 37th week of adjusted age, this possibility has to be considered. The second factor is the presence of vernix, debris and epithelial desquamation in the external acoustic canal (EAC), tympanic membrane abnormalities, middle ear affections, non-absorption of amniotic fluid, partial or total occlusion of EAC by small portions of debris up to the 7th day of life of the newborn10, 21, 22, 23. The factors may result in reduction of intensity or exclusion of sound signal in the EAC. Their presence requires inspection and cleaning of EAC before the application of the OAE test and immitanciometry. Despite being ideal and recommended, at the first stage we did not count on the support of an Otorhinolaryngologist in the nursery to perform such procedure.
We believe that in services without Otorhinolaryngologist in the nursery team, hearing assessment should be performed by the time the child comes to the pediatric visit, normally seven days after the discharge. By then, debris and amniotic fluid have already been naturally absorbed by the middle ear. Thus, the incidence of false-positive would be much lower.
Conversely, parents may prefer to see a pediatrician close to the their own homes or covered by their health plans and that would increase the drop out rate from the program.
The ideal scenario would have pediatric ambulatory services with routine auditory assessment of the newborn, analyzing at what time and what would be the best assessment format according to the economic-financial and cultural conditions of the region. The implantation of a information and awareness program about hearing loss focused on parents, especially in less privileged regions, as suggested by Logan17, would bring benefits to the community. To that end, a national educational campaign directed to parents would raise the importance of early diagnosis.
In our sample, we did not detect suspected cases of sensorineural hearing loss. We found 3.8% (n=7) of newborns with confirmation of suspicion of conductive loss 30 days after birth.
The attendance of 85% of the patients on the second stage enabled the confirmation of 15.5% of false-positive on the 1st stage of assessment.
Prieve et al24 suggested that the minimum return rate for screening follow-up should be 70%. Before the tests were conducted, parents were informed about the importance of hearing for the development of speech, about the possibility of having a false-positive owing to the presence of fluid in the ear and we also stressed the importance of having them back to repeat the tests. Our pediatricians are constantly informing parents about the importance of ambulatory follow-up, especially high risk infants. The examiner also called the parents to explain the importance of reassessing and to emphasize that the babies had already passed in the auditory behavioral assessment. We strongly believe that all procedures together led to the high return rate we noticed in our study, compared to the literature.
As to latency of DPOAE, we did not find statistically significant differences between ears. Our findings are in accordance with those published by other authors17, 22, 25.
Our results showed that mean amplitude of DPOAE are similar in 984, 2.016 and 6.000 Hz and that they are reduced in 3.000 Hz and 3.984Hz. These results were the same for groups GA, GB and GBI. The highest ones were 12.35 dB, 13.26 dB and 12.65 dB for 6.000 Hz for groups GA, GB and GBI, respectively, in agreement with the results found by Coube and Costa Fo, whose mean amplitude value of f2 for 6.000 Hz for the highest one (14.35 dB SPL).
Mean values were high for DPOAE amplitudes in frequency 984Hz, but PD/RF ratio had lower values and higher incidence of failed cases for the other frequencies in the three groups. The results confirmed the recommendation by Gorga26, that suggested not to study f2 at 1000 and 1500Hz in DPOAE because of the poor perception of PD/RF ratio, which increased duration of the test.
We did not find statistically significant differences between the groups concerning mean amplitude of frequencies and PD/RF ratio. Therefore, we believe that cochlear integrity allows similar responses in newborns, regardless of the maternal risk or the hearing loss risk indicator.
We suggest that new studies be performed to collect more information about amplitude of response and PD/RF ratio of DPOAE, especially in newborns with hearing loss risk indicators.
In our sample, even when the newborns were in deep sleep (17.4%) they presented behavioral reactions after hearing stimulation. Lichtig27 also found behavioral reactions in newborns when they were in deep sleep.
The most frequent behavioral reactions observed were: body activity, facial activity and reflex response, confirming the data reported by other authors3, 27, 28, 29.
All subjects passed the auditory behavioral assessment, even those that presented middle ear abnormalities. The results indicated that assessment of auditory behavior through musical instruments was not sensitive to detect this type of alteration. According to the tests, there were no cases suspected of sensorineural loss, so we could not evaluate the efficacy of the method to detect this kind of affection. Alternatively, according to Portmann & Portmann29, the presence of behavioral reactions suggested integrity of auditory pathways and absence of severe or profound hearing loss, reducing parents' anxiety and concerning the presence of a severe loss.
We used PEATE as the gold standard when there was failure in DPOAE after the third stage of assessment. The cases that underwent PEATE showed probable presence of conductive component.
In our study, children submitted to PEATE were sedated. We noticed that parents were concerned about the use of anesthetics, which could explain why some of them did not come to perform the test that required sedation.
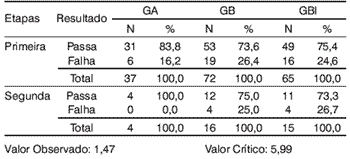
Table 1.
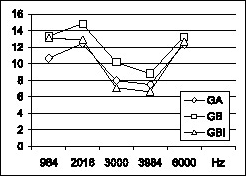
Graph 1. Graphic distribution of mean amplitude (in dB) of Distortion Product in the studied frequencies for GA, GB and GBI.
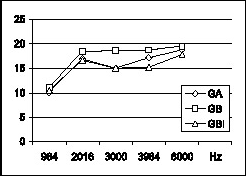
Graph 2. Graphic distribution of mean ratio PD/RF (in dB) in the studied frequencies for groups GA, GB and GBI.
Based on the results found in our sample, we concluded that:
1. DPOAE and auditory behavioral assessment were similar between the groups of babies born to gestational high risk mothers and the control group;
2. Amplitude of DPOAE was similar for the groups of newborns with and without hearing loss risk indicators born to mothers of high gestational risk and the control group;
3. The signal/noise ratio of DPOAE was similar between the group of newborns with and without hearing loss risk indicators born to mothers of high gestational risk and the control group;
4. The method of DPOAE presented high incidence of failures in newborns not submitted to inspection and cleaning of the EAC;
5. Behavioral reaction, after musical instrument stimulation, was similar for both groups of newborns with and without hearing loss risk indicators born to mothers of high gestational risk and the control group;
6. The assessment of auditory behavior using musical instruments was not effective to detect external and/or middle ear affections;
7. DPOAE and auditory behavioral assessment were complementary to assess hearing in the newborn not submitted to inspection and cleaning of EAC or children with possible external and/or middle ear affections.
REFERENCES1. Silveira, JAM. Estudo da deficiência auditiva em crianças submetidas a exames de potenciais evocados auditivos: etiologia, grau de deficiência e precocidade diagnóstica. [Tese Doutorado]. Faculdade de Medicina da Universidade de São Paulo; 1992.
2. Nobrega M. Aspectos diagnósticos e etiológicos da deficiência auditiva em crianças e adolescentes. [Tese Mestrado]. Escola Paulista de Medicina; 1994.
3. Hayes D, Northern J. eds. Infants and hearing. San Diego, CA: Singular Publishing Group Inc., 1996: Foreword; 3-28.
4. White KR, Vohr BR, Behrens TR. Universal newborn hearing screening using transient evoked otoacoustic emissions: results of the Rhode Island assessment project. Semin Hear 1993;14:18-29.
5. Lichtig I, Carvallo RMN. eds. Audição: abordagens atuais. São Paulo. Pró-Fono, 1997:3-22; 45-64.
6. Joint Committee On Infant Hearing. Position statement 1994. Audiol Today 1994;6:6-9.
7. Carvallo RMM, Sanches SGG, Ravagnani MP. Amplitude das emissões otoacústicas transientes e por produto de distorção, em jovens e idosos. Rev Bras Otorrinolaringologia 2000;66(1):38-45.
8. Castro Jr NP, Figueiredo MS. Audiometria eletrofisiológica. In: Lopes Fº O, Campos CH eds. Tratado de otorrinolaringologia. São Paulo: Roca, 1994:638-50.
9. Joint Committee On Infant Hearing. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. American Journal of Audiology June 2000;9:9-29.
10. Lopes Fº O, Carlos R, Thomé D, Eckley C. Emissões otoacústicas transitórias e produtos de distorção na avaliação da audição em recém-nascidos com poucas horas de vida. Rev Bras Otorrinolaringologia 1996;62(3):220-8.
11. Northern JL, Downs MP. ed. Hearing in children. 2nd ed. Baltimore, Maryland: The Williams & Wilkins Company; 1979.
12. Helander E. Prejudice and dignity. An introduction to community-based rehabilitation, Geneva, World Health Organization, 1993.
13. Russo ICP. Overview of audiology in Brazil: state of the art. Audiology 2000;39:202-6.
14. Dell'aringa AR. Contribuição ao estudo das disacusias hereditárias, progressivas e de causas desconhecidas. [Tese Doutorado]. Faculdade de Medicina da Universidade de São Paulo; 1999.
15. Matas CG, Sansone AP, Iorio MCM, Succi RCM. Avaliação auditiva em crianças nascidas de mães soropositivas para o virus da imunodeficiência humana. Rev. Bras. Otorrinolaringolologia 1999;66(4):317-24.
16. Coll J, Régnier CL. Les potenciels auditifs précoces dans la dépistage de la surdité. Revue de Laringologie 1988;109:325-6.
17. Logan S. Early identification of impairments in children. In: Zinkin P, Mcconachie H. ed. Disabled children and developing countries clinics in development medicine. 1995;136:101-9.
18. Amatuzzi MG. Contribuição ao estudo das células ciliadas da cóclea de recém-nascidos de alto risco: histopatologia post-mortem. [Tese de doutorado]. Faculdade de Medicina, Universidade de São Paulo; 1997.
19. Andrade MH, Oliveira JAA. Contribuição ao estudo da deficiência auditiva em crianças. Rev Bras Otorrinolaringologia 1992;58(4): 2725.
20. Bassetto MCA. Emissões otoacústicas evocadas transientes: estudo da amplitude de resposta em recém-nascidos a termo e pré-termo. [Tese Doutorado] Universidade Federal de São Paulo - Escola Paulista de Medicina; 1998b.
21. Eavey RD. Abnormalities of the neonatal ear: otoscopic observations, histologic observations, and a model for contamination of the middle ear by cellular contents of amniotic fluid. Laryngoscope 1993;103:131.
22. Hall JW. ed. Handbook of otoacoustic emissions. San Diego, CA: Singular Publishing Group, 2000.
23. Robinette MS, Glattke TJ. ed. Otoacoustic Emissions. New York: Thieme. 1997:83-109; 130-50.
24. Portmann M, Portmann C. Tratado de Audiometria Clínca. 6ª ed. São Paulo: Roca. 1993; Cap. 14:268-87.
25. Coube CZV, Costa Fo AO. Emissões otoacústicas evocadas: produto de distorção em indivíduos com audição normal. Rev. Bras. Otorrinolaringologia Jul/Ago, 1998;64(4) Parte 1:339-46.
26. Gorga MP. Curso: Aplicações clínicas das emissões otoacústicas e BERA. [apostilas] São Paulo, SP. setembro 2000.
27. Lichtig I. As respostas de neonatos a diferentes tipos de estímulos auditivos. Cadernos de Distúrbios da Comunicação. Série Audiologia 1984:3.
28. Azevedo MF, Vieira RM, Vilanova LCP. Desenvolvimento auditivo em crianças normais e de alto risco. São Paulo. Plexus, 1995:11-4.
29. Pialarissi PR, Gattaz G. Emissões otoacústicas: conceitos básicos e aplicações clínicas. Arquivos da Fundação Otorrinolaringologia 1997;1(1):1-3.
1 Audiologist, Master in Sciences, Medical School, USP, Coordinator of Specialization Course on Audiology, Medical School, ABC.
2 Associate Professor, Head of the Department of Speech and Language Therapy and Audiology, Physical Therapy and Occupational Therapy, Medical School, USP.
3 Ph.D., Professor, Discipline of Otorhinolaryngology, Medical School, USP.
Summary of the Master Dissertation Thesis, Medical School, USP - Area of Experimental Pathophysiology, approved on August 01, 2001.
Address correspondence to: Marisa Ruggieri Marone - R. Rene Zamlutti, 160 ap 52 - CEP 04116-260 - São Paulo - Tel/fax: (55 11)5539-3020 - E-mail: mruggieri@uol.com.br
Article submitted on October 15, 2001. Article accepted on January 17, 2002.


