IntroductionNasal obstruction in childhood is an important complaint in the Otorhinolaryngological practice. In this period of life, it is necessary to be concerned about the affection because it is the time the face and the nose grow and develop. Among the most significant causes of nasal obstruction in childhood we may list septal deviations, which affect about 20% of the population. In 25% of those who have this affection it is responsible for a significant resistance to the airflow1. The septal surgery in childhood started as an attempt to restore normal nasal function, help normal nose development, prevent underdevelopment of columella and prevent deformities of the nose tip2. At the beginning, the main surgery to correct nasal septal deviations was septal submucous resection, created by Killian & Free1. We used to think that the surgery destroyed the so-called growing mechanisms of the septal cartilage, impacting the growth of the nasal septum and face, leading to postponement of the surgery up to the age of 15 or 16 years. There were surgeons against this principle2,3,4,5,6,7,8,9,10,11,12 and in favor of it 13,14,15,16,17,18,19,20. Therefore, many studies dedicated to identifying the growth centers of the septal cartilage in order to reduce the postoperative problems and then, make it possible to correct deformities before puberty. Our study intended to define the behavior of different regions of nasal quadrangular cartilage of human nose during growth, suggesting implications in septoplasty intervention in children.
Material and MethodWe removed 44 septal cartilages of human cadavers submitted to necropsy. The sample consisted of male Caucasian subjects aged 5 days to 66 years, totaling 12 cartilages. The septal cartilage was removed in block with the whole cartilaginous framework through open rinoplasty approach, as advocated by Goodman21,22,23,24 and by Anderson25. We created a technique of nasal pyramid reconstruction, using cardboard paper and cotton as raw materials. The incision suture was performed with chromium 0000 cat gut, and it did not leave any post-resection nasal deformity. The pieces that were removed were immediately fixed in a solution of formalin at 10%. Once recognized the anatomical relations, the pieces were finished with the lamina of the knife by defining two planes of frontal sections: one on the causal and middle thirds and another on the transition of middle and cephalic thirds. The result was the definition of a three-piece block: caudal third (position 1), middle third (position 2) and cephalic third (position 3). In turn, each position was subdivided into sites in the anterior-posterior axis: upper anterior third (site 1), middle third, center (site 2), lower posterior third (site 3). Thus, the three different positions were divided into three different sites and constituted 9 regions for the studied cartilages (Figure 1).
Histologic preparation: after 24 hours of embedding into fixing solution, the pieces were submitted to dehydration in a battery of ethanol growing concentration solutions (50%, 60%, 70%, 80%, 90%, 95%, 100% and 100%) and diaphanized in a solution of benzene plus ethanol (1:1 v/v), benzene 100% and included in paraffin. From the pieces, we obtained blocks that were cut in 7-mm thick slices, which were placed in glass slides, deparaffined with a xylol solution and rehydrated immersing the slides in xylol plus ethanol (1:1 v/v), in batteries of decreasing ethanol concentration (100%, 100%, 95%, 90%, 80%, 70%, 60%). After rehydration, half of the slides of each position and site were stained by hematoxylin-eosin (HE) and the other half was stained with Alcian Blue. The slides containing sections were rehydrated following the opposite order of hydration, diaphanized with benzene and prepared in permount.
Three studies were applied:
1. Histologic analysis: we performed it in the slides stained with HE, using Zeiss microscope, 40X objective. We concentrated on observing growth found in the cartilaginous tissue.
2. Optic densitometry: we performed it in the slides stained with Alcian Blue in order to determine the variations of cartilaginous matrix. This stain interacts with the polyonic sulfate glucoaminoglycans present in the matrix26, and therefore, their concentration reflected the metabolic activity of the cartilage; this activity could be related to the growth phenomena. Optic densitometry of Alcian Blue may convey notions of relative concentrations between the various regions and the various ages within the same region by the application of Beer-Lambert Law. The law is based on the fact that in media in which the substance is homogeneously diluted, absorption of monochromatic light will directly depend on the length of the light path in the solution and on the concentration of the solute and will inversely relate to the light projection area. Once we have fixed the length of the path and the projection area, the absorbance of the monochromatic light will be proportional to the concentration of the studied substance, according to the following formula:
a = log I/I0
Where: a = absorption
log = logarithm in base 10
I = intensity of detected light with object interposition
I0 = intensity of detected light without object interposition.
Absorbance is not linearly related to amount of light absorbed by the substance of interest and, therefore, heterogeneous media such as those found in histologic preparations are subject to distribution errors. Thus, upon considering the light intensity in an area heterogeneously stained in a preparation, the value 1 will be the mean of light intensity values in the innumerous points of the previous sums. The easiest way to correct such a error is to divide the region in innumerous small regions in which heterogeneity is small, take the absorbance value of each one of them and calculate the mean of all obtained absorption values. The software Image tool from University of Texas Health Science Center at San Antonio was the software used for optic density measures. The calibration standard of linear distances was the Zeiss slide graduated in lines of 10 mm. Optic densities were taken from the cartilaginous matrix in each position and site of the subperichondral region of one side to the subperichondral region of the other side, using number 9 Zeiss filter as monochromatizer. The measures of I0 were calculated by measuring the light intensity present in regions of the slide that had no sections. As to the comparison of optic density, we calculated linear regression analyses in each position and each site, considering subjects' age as an independent variable.
3. Morphometric study: The morphometric measures were performed in the slides stained with HE. The image recording and analysis system was the same used for optic density measures. We measured the thickness of cartilage and perichondrium in each of the positions and the respective sites and length of the septum in the three positions. We made 10 measures in each one of the regions and then calculated the mean values. In the analysis of cartilage thickness variation we applied logarithm regression in the obtained values, because this function was the one that best adapted to most cases.
The logarithmic function is represented by:
e = a ln i + b
Where: e is the thickness observed in the cartilage.
a and b are constants.
i is the age in years.
After obtaining the equations that represented cartilage thickness in relation to age in years, we calculated the speed of increased thickness simply by using the function derived from the logarithmic formula obtaining v = a i-1, where v = speed of increase of cartilage thickness, a is the same constant and i is age in years. In the analysis of variation of perichondrium thickness, we applied grade 3 polynomial regression because this function was the one that adapted the best to most cases. Grade 3 polynomial function is represented by e = a x i3 + b x i2 + c x i + d, where: e is the perichondrium thickness, a, b, c and d are constants, i is age in years. Therefore, the obtained curves represented always a maximum point. The ages in which the maximum thickness of the perichondrium were reached are those in which the first derivative of the function obtained above is equal to zero and the second derivative has value below zero. Since it is a grade 3 polynomial function, it always admits first and second derivation from which there are two equations: The first derivative is defined as e' = 3 x a x i2 + 2 x b x i + c and the second as e" = 6 x a x i + 2 x b, where a, b, c and i are the same variables of the regression polynomial.
In the comparison of values of quadrangular cartilage thickness and length, the points were adjusted for a curve of logarithmic regression and we considered points of reduction of velocity of growth related to age in which the derivative curve obtained was equal to 1. The software used in all statistical analysis and for the generation of the graphs was Excel version 7 by Microsoft.
Results1. Observation of histology of septal cartilage: In very young cartilages, such as in nine-month-old subjects, we observed a great number of young cells in the periphery and very clear zone of transition; there was a great number of mature cells in the center and the formation of few isogenous groups (Figure 2, region P1L1). In six-year-old subjects, when we expect slower velocity of growth, we observed characteristics of a more mature cartilage: few cells in the periphery, poorly defined transition zone, lower amount of cells in the cartilage center and greater number of isogenous groups (Figure 3, region P1L2). The same applies to subjects aged 12, 15 and 37 years.
2. Optic densitometry: The optic densitometry reduced significantly in all regions, except for site 2, position 2, which corresponded to the geometric center of the cartilage (Table 1). There were no statistically significant differences between angular coefficients in the regions where there was optic density reduction or in the values of interceptor (Figures 4, 5 and 6).
3. Morphometry: The parameters of the logarithmic curve, applied to the measure of cartilage thickness, as well as the levels of significance, are presented in Table 2 (Figures 7, 8 and 9).
It is important to note that the logarithmic model was not adjusted for regions P2L2, P3L1, P3L2 e P3L3, and it was not possible to use it to infer about cartilage thickness at different age ranges. Based on these parameters, it was possible to infer the mean expected values for cartilage thickness for ages 1 to 15 years and the values for velocity of growth expected for the same ages (Table 3).
The speeds in red are those in which growth in one year was higher than 2% of the thickness, in blue are those in which growth was higher than 1% of thickness and in green are those whose growth was lower than 1%. The speeds in gray are those of the sites and positions in which the adjustment by the logarithm was not significant, or in other words, it was not possible to infer the speed of thickness increase.
Over the values of mean length measures of quadrangular cartilages in each position, we applied the logarithmic regression analysis that showed that this regression model is well adjusted to the study of cartilaginous septum length related to age (Table 4 and Figure 10).
Based on such parameters, it was possible to infer the expected mean values of length for all ages between 1 and 15 years and the velocity values for length increase expected for the same ages (Table 5).
Over the values obtained on measures of perichondral thickness in each position, we applied grade 3 polynomial regression analysis which was the one that adjusted the best in the study of perichondral thickness variation in relation to age (Table 6).
The following were significant at 1% level - P1L1, P1L3, P2L3 and P3L1, whereas P1L2 was significant at the level of 5%. With such parameters, it was possible to determine at what ages, in each position and site, the perichondrium reached its maximum thickness (Figures 11, 12 and 13).
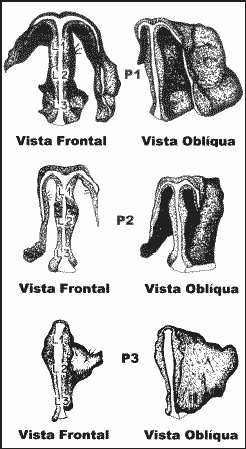
Figure 1. Regions that define the positions and sites of the study.
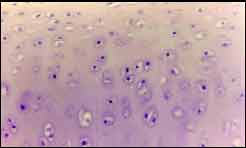
Figure 2. Nine-month-old subject. Transition zone, mitosis figures and cell hypertrophy.
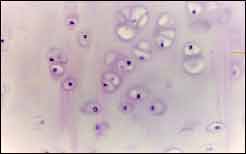
Figure 3. Six-year-old subject, cellular hypertrophy, formation of isogenous groups.
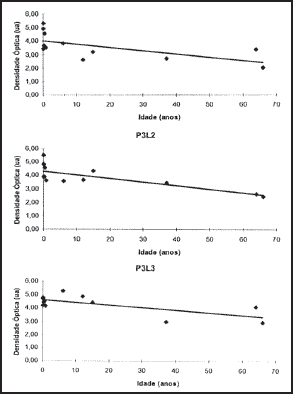
Figure 4. Values obtained and regression straight lines in position 1.
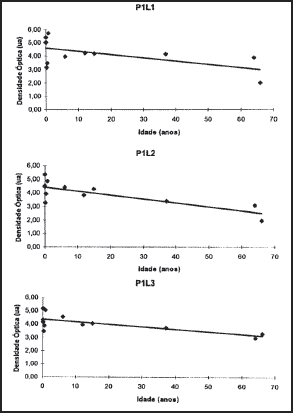
Figure 5. Values obtained and regression straight lines in position 2.
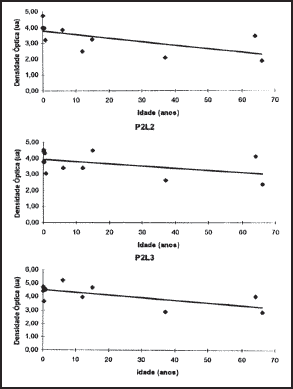
Figure 6. Values obtained and regression straight lines in position 3.
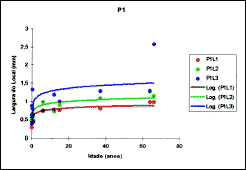
Figure 7. Values obtained for mean thickness of quadrangular cartilage in position 1.
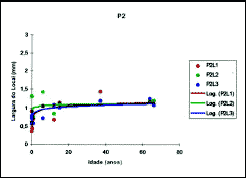
Figure 8. Values obtained for mean thickness of quadrangular cartilage in position 2.
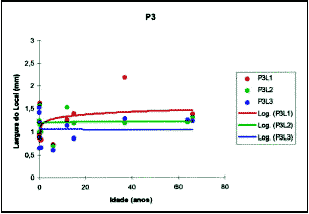
Figure 9. Values obtained for mean thickness of quadrangular cartilage in position 3.
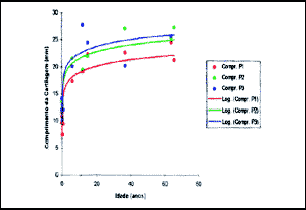
Figure 10. Values obtained for mean length of quadrangular cartilage in positions 1, 2 and 3.
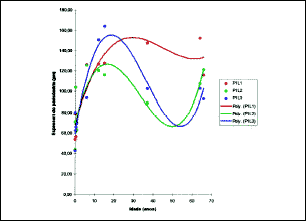
Figure 11. Values obtained for men length of perichondrium in P1L1, P1L2 and P1L3. The circles represent the observed values and the lines are the curves inferred for grade 3 polynomial regression.
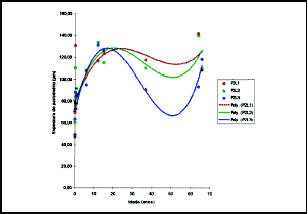
Figure 12. Values obtained for mean thickness of perichondrium in P2L1, P2L2 and P2L3. The circles represent the observed values and the lines are the curves inferred for grade 3 polynomial regression.
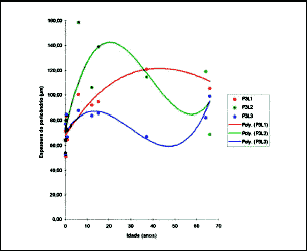
Figure 13. Values obtained for mean thickness of perichondrium in P3L1, P3L2, and P3L3. The circles represent the observed values and the lines are the curves inferred for grade 3 polynomial regression.
The identification of high growth potential areas of the quadrangular cartilage or the "growth centers" are the main concern to rhinosurgeons, because once they know these areas, surgeries may be performed more safely. Similarly, the knowledge about septal growth pattern is essential, because once we know how the tissue develops, we can determine the most appropriate age to intervene. In summary, it is crucial to answer the following questions: As of what age is it safe to operate on? Which area of the quadrangular cartilage is more vulnerable to surgical intervention? Does septal surgery in childhood, even with conservative technique, affect nasal growth? Should we really wait until 15 to 16 years to perform a septoplasty? Mosher (apud Vetter)27 advocated the existence of two growth centers in the human septal cartilage after the age of 8 years: the first would be located above pre-maxilla area and the second on the upper half of the posterior septal border. Klaff28 reported that in the region of pre-maxilla ala the quadrangular cartilage is thin and it is also its key growth center. White13 pointed as growth center the junction of the inferior lateral cartilage and the septal cartilage and the junction of the septal cartilage with the perpendicular plate of ethmoid. Our histologic findings showed a 6-year-old subject whose growth histologic characteristics were less clearly defined than in younger subjects, such as in a 4-month and a 9-month subjects: there are few cells in the periphery, less exuberant transition zone and formation of isogenous groups disperse in the cartilage center, similar to subjects who are 12, 15 and 37 years. The metabolic activity in the matrix suffers continuous and regular drops with aging, except for one case (P2L2), whose linear decrease happened from birth to advanced age. Apparently, region P2L2, since it holds the most central portion of the cartilage, should reach faster the levels of maturation compared to the other regions. Studies by Vetter et al27,29 using in vitro techniques showed differences in metabolic activities in different regions of the nasal septum, showing age-dependent decline and regions that had to be preserved or be minimally resected, as the septum anterior free margin, supra pre-maxilla and central regions. To assess metabolic activity of the matrix, we used different methods from the ones described by those authors, since our study was conducted in vivo. Our findings did not show significantly different metabolic activity of the cartilage in any studied area in different ages, but we also noticed linear decrease of the metabolic activity with aging, which was high in childhood, lower in adolescents and much lower in adult subjects. Based on our findings, surgical resections could be conducted similarly at any area of the cartilage, since we did not detect differences in metabolic activity among the different studied positions.
As to cartilage thickness, at least in 5 regions of the septal cartilage it was possible to record a curve of expected values of cartilage thickness by age. The curve described the behavior of all thickness measures by position. All curves of septal thickness values in the three positions were adjusted with significance level of 5%. The velocity of growth in all ages contained in the interval 0 to 15 years was obtained from the derivation of the growth for each age. Knowing that most deviations are situated in the caudal region and that thickness velocity of growth smaller than 1% is reached after 9 years of age, septum surgery can be more safely performed from 9 to 15 years for the caudal, central (superior) and central (inferior) regions, since the low thickness velocity of growth is found in such areas. In situations in which speed is higher than 1%, with ages ranging from 5 to 8 years, we should perform the surgery with care, since in this period the septal cartilage is still under considerable growth, especially in the caudal and anterior central (superior) regions. The growth of septal cartilage has an active role in facial growth, pushing the maxilla forward and downwards30. Other authors believe that the septum plays a passive role in facial growth. This disagreement generated doubts about the surgical intervention in the septum of subjects in the growth stage. Our results showed that septum length velocity of growth is greater in the age of one to three years in the 3 studied positions and it reduces with aging, although it is still significant at the age of 5 years. Thus, it seems that surgical interventions should be performed after the age of 5 years, when septal length velocity of growth is reduced. However, it is after the age of 8 that velocity of growth reaches the lowest values, a fact that indicates it is the best age to start septal surgical interventions. Our considerations are in agreement with those made by Olsen et al18 who advocated that an intact nasal septum is necessary for the normal facial development. The authors preferred reconstruction procedures instead of radical resection and they noticed that no modification of nasal growth took place postoperatively. Similarly, Jennes & Conn17 stated that conservative surgeries with minimal resections showed normal nose development in the postoperative follow-up. The perichondrium growth was not similar to the growth of septal cartilage thickness and length. The maximum height varied according to position, site and age. Therefore, the behavior of the perichondrium concerning increased thickness should not be the main parameter over age for surgical intervention. We noticed that minimum age with the thickest perichondrium was observed at the age of 13 years for cephalic position (posterior), at 17 years for middle position and at 16 years for causal position. The importance of surgical preservation concerns the nutritional role of the septal cartilage. Septum surgery may be performed as of the age of 6 years and it is more safely performed at the age of 8 and 9 years. The dictum "wait until the patient turns 16 or 17 years to perform septal surgery"14 may seem to be an overprotection measure, most probably owing to the fear of complications, especially because of lack of surgical expertise or the use of more radical surgical methods.
ConclusionThe morphometric study of septal cartilage thickness and length from 2 days to 66 years of age showed greater velocity of growth in the first 5 years of life. The velocity reduces with time. The study of metabolic activity of the cartilage using densitometry showed that the tissue presents similar behavior in all its extension, which is higher in the first years of age. Based on such findings, we believe that surgical interventions may be performed after this period, if necessary. However, the most indicated period for such surgeries starts after the age of 8 years.
References1. Blaugrund SM. The Nasal Septum and Concha Bullosa. Otolaryngol Clin North Am, 1989;22(2):291-306.
2. Metzenbaum M. Asymmetry of the Nares: A Positive Diagnostic Sign or Entity Establishing Anatomic Displacement of Lower End of Cartilaginous Nasal Septum. Arch Otolaryngol, 1932;16:690-7.
3. Metzenbaum M. Dislocation of the Lower End of the Nasal Septal Cartilage. Arch Otolaryngol, 1936;24:78-85.
4. Cottle MH. Nasal Surgery in Children. EENT Monthly, 1951;30:32-8.
5. Cottle MH & Loring RM. The Maxilla-premaxilla Approach to Extensive Nasal Septum Surgery. Arch Otolaryngol, 1958;68:301-13.
6. Gilbert JG & Segal SL. Growth of the Nose and the Septorhinoplastic Problem in Youth. Arch Otolaryngol, 1958;68:673-82.
7. Gray LP. The deviated nasal septum I aetiology. J Laryngol, 1965;79:565-75.
8. Montserrat JM. Cirurgia Funcional de la Nariz em la Infância. Acta Orl Iber Am, 1968;2:150-62.
9. Stucker FJJR, Bryarly RC, Shockley WW. Management of Nasal Trauma in Children. Arch Otolaryngol, 1984;110:190-2.
10. Jugo S. Septal Surgery in Children. Symp Otorhinol Iug. 1986;21(23):29142.
11. Stool SE. Postnatal Craniofacial Growth and Development, in Bluestone CD & Stool SE. Pediatric Otolaryngology, Philadelphia, WB SAUNDERS, 1983;1(2):18-30.
12. Montserrat-Viladiu JM & Montserrat-Gali JR. Septoplastia Infantil. F Med (BR), 1987;95(2):83-92.
13. White FW. Submucous Resection of the Nasal Septum in Children, Arch Otolaryngol, 1930;11:415-25.
14. Salinger S. Injuries to the Nose in Children. Arch Otolaryng, 1941;34:93651.
15. Lefkon IM. Rhinoplasty in Children. Arch Otolaryngol, 1948;48:7382.
16. Fine IJ. Goldman technique in nasal septal surgery. Arch Otolaryngol, 1954;54:141-6.
17. Jennes ML & Conn W. Corrective Nasal Surgery in Children; Arch Otolaryngol, 1964;79:145-51.
18. Olsen HD, Carpenter RJ, Kern EB. Nasal Septal Injury in Children, Arch Otolaryngol, 1980;106:317-20.
19. Fior R & Veljak C. Septum dislocation in the newborn: a log term follow up study of immediate reposition. Rhinology, 1990;28:159-62.
20. Walker PJ, Crysdale WS, Farkas LG. External Septorhinoplasty in Children: Outcome and Effects on Growth of Septal Excision and Reimplantation, Arch Otolaryngol Head Neck Surg, 1993;119(9):984-9.
21. Goodman WS. External Approach to Rhinoplasty. Can J Otolaryngol, 1973;2:207-10.
22. Goodman WS & Charbnneau PA. External Approach to Rhinoplasty. Laryngoscope, 1974;84:2195-201.
23. Goodman WS & Charle DA. Technique of External Rhinoplasty J Otolaryngol, 1978;7(1):13-7.
24. Goodman WS. Recent Advances in External Rhinoplasty. J Otolaryngol, 1982;10:433-9.
25. Anderson JR, Johnson CM, Adamson P. Open Rhinoplasty: An Assessment. Otolaryngol Head Neck Surg, 1982;90:272-4.
26. Hall BK. Cartilage. Structure, Function and Biochemistry. USA, Academic press, subs. Harcourt Brace Jovamovich Publ., The Chondroblast and the Chondocyte, 1983;1(3):59-78.
27. Vetter V, Heit W, Helbing G, Heinze E, Pirsig W. Growth of the Septal Cartilage: Cell Density and Colony Formation of Septal Chondrocytes. Laryngoscopy, 1984;94:1226-9.
28. Klaff DD. The Surgical Anatomy of the Antero-Caudal Portion of the Nasal Septum: A Study of the Area of the Premaxilla. Laryngoscope, 1956;66(7):995-1020.
29. Vetter V, Pirsig W, Heinze E. Growth Activity in Human Septal Cartilage: Age Dependent Incorporation of Labeled Sulfate in Different Anatomic Location. Plast Reconstr Surg, 1983;71(2):167-71.
30. Scott JH. The Cartilage of the Nasal Septum, Brit Dent J, 1953;95:3743.
Private Practice
Address correspondence to: Rua Siriri, 1024 - Tel.: (55 79) 214-3748 - Fax.: (55 79) 211-7413 - e-mail: ccose@bol.com.br
Presented at Congresso Triológico da SBORL - Goiânia - Brazil - August 2001. Awarded with the 1st prize in Rhinology.
Article submitted on December 06, 2001. Article accepted on December 20, 2001


