INTRODUCTIONThe management of tracheal and subglottic stenosis is still a controversial issue these days and tracheotomy, which is one of the treatments immediately used, is not a risk-free procedure. Some of its risks include bleeding, pneumothorax, tube obstruction due to secretion, tracheal extrusion of the cannula, difficulty to cry, speak and high mortality rate (2 to 24%)1-4.
Another surgical treatment is laryngotracheoplasty and it is the incision of cricoid cartilage and of the second and third tracheal rings and the placement of a tracheal stent for two or three weeks. Success rate of this technique used to be 75%3-5.
Prescott6 developed the same technique, but with the placement of a graft of costal cartilage. According to Prescott et al. 8-19, this type of procedure enabled early extubation with no need to use stent, and with increased success rates of 70 to 80% of the cases.
Other procedures involve tracheal resection and termino-terminal anastomosis20-25. These surgeries may be required after surgeries due to trauma, cancer or inflammation, tumor or post-intubation stenosis. Generally, 6 to 8 cm resections could be repaired with termino-terminal anastomosis alone or combined with prosthetic devices, or nasal cartilage, costal and tracheal grafts 5, 6,10,28,30. Frequently, in these surgeries columnar ciliated epithelium is replaced by squamous epithelium with presence of epithelial metaplasia in certain areas and granulated tissue formation obstructing tracheal lumen18, 26, and 27. The association with such epithelial regenerative alterations and tracheal cartilage absorption may result in circumferential stenosis formation, and further respiratory obstruction. The resulting stenosis may require other surgeries, such as dilations or silicone or metal stenting 28. Notwithstanding these techniques, we still have increased complication potential such as bleeding, collapse or displacement of the stent, lumen reduction, inflammation and granular tissue formation. 28-30. It is understood, however, that reconstructive tracheal surgeries using alloplastic or tissue materials theoretically cause decreased inflammatory reaction ensuring at the same time proper tracheal lumen, which are object of ongoing studies10-18. The present study intended to observe regenerating characteristics of tracheal epithelium, occurrence or not of cartilage reabsorption and proper tracheal lumen in tracheal transplant procedure.
MATERIAL & METHODSFifty-one guinea pigs (Cavia Porcellus) with variable age, weight and gender were randomized in groups according to observation time of the animals (table 1). Animals underwent surgery after intraperitoneal anesthesia with pentobarbital (50 mg/kg of weigh). Under aseptic conditions, the cervical trachea was exposed by a median line incision after separating the muscles and pre-tracheal fascia. Repair sutures were performed on the anterior tracheal wall above and below the segment to be excised. After resecting six tracheal half-rings, the homograft was placed and sutured with single synthetic (nylon) thread 8.0, termino-terminal suture, separated stitches, with inferior anastomosis performed first.
Tracheal homograft was collected from guinea pig with age and weight similar to those of animals of the transplanted group. The cervical trachea was excised, with removal of an average of 6 to 8 tracheal rings. The preparation and preservation technique of the graft was the following: washing with saline solution, removal of respiratory and soft tissues epithelium and conservation in formaldehyde 4.5% pH 5.6 for 2 weeks. Homograft was washed and soaked in saline solution for at least 2 hours, a method that has been described in details in the literature 4, 10, 11, 14-17.
Animals were kept in cages and sacrificed according to the Schedule shown in Table 1. Five animals were excluded from the study because they died on the first post-operative day due to respiratory depression. After sacrificing the animals, histological tracheal fragments were prepared with hematoxylin-eosin dying, Masson Trichromic Acid, and Schiff Periodic Acid (PAS). Cross-sectional and longitudinal histological slices were prepared. Aspects studied were as follows: tracheal lumen, transplanted area, repithelization, cartilage reabsorption and inflammatory reaction.
TABLE 1: Tracheal transplant in guinea pigs. Survival time in days. Weight of animals. Metaplasia. Tracheal stenosis.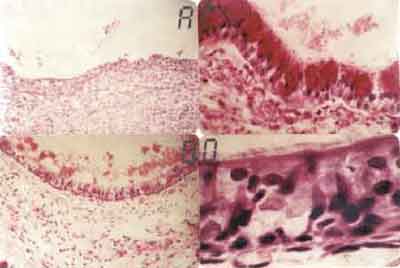
Figure 1. A. Neutrophilic inflammatory reaction in the corium, submucosa and perichondrium, attacking and desquamating the tracheal epithelium (HE - 31X). B. Further magnification of the previous field, showing in details the neutrophilic inflammatory reaction affecting and damaging the epithelium. We can observe the cartilage that has an hyaline aspect and chondrocytes with effaced nuclei (HE-500x). C. Squamous epithelium formed by 1 to 3 cell layers. Cartilage calcification area shown in black (HE-320X). D. Further magnification of the previous field showing in details the squamous epithelium formed by 1 to 3 cell layers (HE 640 X).
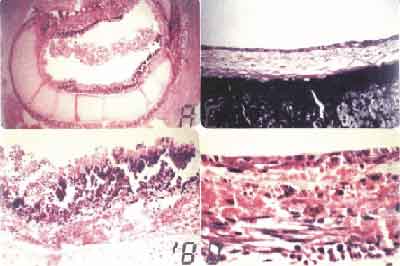
Figure 2. A. Area of squamous metaplasia and reaction epithelium (HE - 200X). B. Respiratory epithelium showing goblet cells and loose connective tissue in the submucous layer (HE-192X). C. Respiratory epithelium stained with PAS, showing in red the mucous produced by the goblet cells (PAS - 1250 X). D. Ciliated cylindrical epithelium, goblet cells and reserve cells (HE-1600X).
RESULTSWith 3-day survival animas, fibrin-leukocytarian exsudate was found in tracheal lumen with desquamated epithelium, detaching from tracheal wall. Severe neutrophilic inflammatory process involving corium and sub mucosa extending to adventitia and peritracheal soft tissues, including recipient trachea (Fig. 1A and 1B). Near the anastomosis site of the recipient area, new squamous metaplasia foci could be observed in 7 out of the 9 animals in the group. Epithelium was reactive, formed with cells with bulky and hyper-pigmented nuclei with evident nucleoli. Compact fibrin-leukocytarian exsudate was found after 7 days, forming sometimes a tracheal stopper. Reactive squamous epithelium formation was observe on the graft next to the anastomosis, with basal cell and granulation tissue hyperplasia and some huge foreign-body type cells around suture threads. In sub-group 3 animals (14 days), lining epithelium of the graft was formed with 1 to 3 cell layers filling almost all transplanted area (Fig. 1C and 1D). Two animals presented goblet cell formation and ciliated epithelium areas near anastomosis.
In animals of late observation (30 days), partial stenosis of the donating trachea, at variable degrees, was reported, but they presented proper tracheal lumen. In seven animals, graft was totally lined with respiratory epithelium and two had squamous epithelium with 3 cell layers in central areas of the anastomosis. In the areas with reactive epithelium, (epithelial metaplasia) inflammatory infiltrate was neutrophilic with rare corium and sub mucosa lymphocytes. (Fig. 2A). Even in those areas where lining epithelium of the graft was respiratory, there was intense cell proliferation, with corium and sub mucosa vascular congestion. Granulomatous reaction formed by histiocytes and gigantic cells with cartilage reabsorption foci was observed around the suture threads in anastomosis site.
In the 60-day subgroup animals, lining epithelium of the grafting was respiratory, with innumerable goblet cells and cilia present (Fig. 2B). Three animals presented sub-epithelial glands in the middle of dense connective tissue lined with simple pseudostratified cylindrical epithelium with goblet cells. Three other animals presented squamous metaplasia foci, cartilage reabsorption and variable degrees of tracheal disintegration and partial graft stenosis in the anastomosis site. The 120-day observation animals had young fibroblasts in the corium and sub mucosa with formation of elastic fibers amidst dense connective tissue. Epithelium was cylindrical, ciliated with innumerable goblet cells producing secretion. (Fig. 2C and 2D).
DISCUSSIONOne of the criteria to evaluate proper tracheal prosthetic procedure is quality of repithelization of transplanted trachea5, 12. Several authors described epithelial regenerating characteristics and its origin and formation of tracheal granulation tissue 3,7,8,13,26,27. In the opinion of MORFITT et al. (1955)8, epithelial regeneration occurs from the anastomosis site to surrounding soft tissues and from recipient trachea. From the first days to the third week multi-stratified cuboid epithelium is observed. ATAMANYUK and MELROSE (1965)15 described these histological changes, such as epithelial metaplasia and squamous epithelium, as evolution steps of epithelial regeneration that come after the phases in which aggression is higher and tend to disappear in the steps when tissue repair is dominant17. Other changes such as basal cell hyperplasia and gland increased secretion, also seem to be present in the beginning of epithelial regeneration, and are closely related to surgical aggression and repair phases of any tissue injury 17,18,26,27.
We observed these changes in all animals. During the first days until the first week occurred intense neutrophilic inflammatory process that attacked and exfoliated transplant epithelium. Subsequently, however, in the second week repair phase started with formation of squamous 1 to 3 cell layers of epithelium near anastomosis site with further evolution to respiratory epithelium. In 30 days, transplanted tissue epithelium did not present almost any signs of severe inflammatory activity, except the observation of large amounts of goblet mucin-producing cells. Persistence of such cells is a symptom of residual inflammatory activity and mucous formation obstructing airways would explain many of the complications and deaths that occur during those post-operative middle and late phases1,2,3,6,10,17,18.
During the period four to six weeks after transplant procedure the replacement of cartilage absorption areas, near anastomosis site, by dense connective tissue begins. These areas are more delicate and decreased tracheal lumen can be observed there (stenosis). According to some authors, these alterations (chronic inflammatory process, scarring tissue and stenosis) could be associated with transplanted tissue rejection. In these cases the use of immunosuppressive drugs, including steroids or even splenectomy would be useful as therapeutic measures to delay or decrease rejection intensity 20-23.
This study did not use any immunosuppressive technique to minimize rejection or to decrease inflammatory process because in our view more than rejection itself, scarring tissue formation was caused by (1) cartilage impairment due to graft conservation technique, or (2) due to trauma of the surgical technique used. Even after carefully following the recommendations of Maeda and Grillo24, to use suture threads with mini-needles under microscopic techniques and prosthetic devices with a maximum of six tracheal rings to decrease tension over the anastomosis, we found out that fragmentation and partial necrosis occurred in transplanted and recipient tracheal cartilage, which was replaced by dense connective tissue.
This was also observed by other authors in large follow-up clinical trials 20-23. Tracheal cartilage was totally replaced by collagen with partial or late circumferential formation of stenosis eight to twelve months after transplant procedure. In these cases, due to the decrease of tracheal lumen, respiratory distress and even death of the animals could be expected. In these studies, however, as in our study, animals did not present stridor and had normal development including weight gain. How could we explain that in spite of reabsorption and cartilage fragmentation tracheal lumen was satisfactory? For some authors, and we agree with them, tracheal lumen permeability would be explained by the appearance of elastic fibers in the corium and sub mucosa of transplanted trachea, mainly in animals with survival rate from 60 to 120 days 19-23. These elastic fibers would be partially responsible for variations in length, diameter and position of normal trachea during breathing, swallowing and movements of the neck, and they would also be able to keep tracheal lumen, if not completely, at least two thirds of it. Other not excluding factors would be collagen tissue formation and major calcification of transplanted cartilage.
CONCLUSIONAlthough graft repithelization occurs, persistent residual inflammatory process in late phase of transplants (120 days) and the scarring tissue formation with consequent stenosis, show that the aspects related to keeping tracheal lumen and tissue rejection deserve new studies before definitive inclusion of this technique in repair surgeries of human trachea is made.
REFERENCES 1. Flemming i, Hommerich kw. Tracheal stenosis development and the latest state of experimental surgery. J Fr Oto-Rhino-Laryngol 1974;23:387-92.
2. Cotton RT, Gray SD, Miller RP. Update of the cincinnati experience in pediatric laryngotracheal reconstruction. Laryngoscope 1989;99:1111-1116.
3. Grillo HC. Reconstruction of the trachea: experience in 100 consecutives cases. Thorax 1973;28:667-78.
4. Hoffer ME, Tom LW, Wermore, RF, Handler SD, Potsic WP. Congenital tracheal stenosis. The otolaryngologists perpectives. Arch Otolaryngo. Head Neck Surg 1994;120:449-53.
5. Cotton RT, Myer CM III, O´Connor DM, Smith ME. Pediatric laryngotracheal tube reconstruction with cartilage grafts and endotracheal tube stenting: the single-stage approach. Laryngoscope 1995;105:818-21.
6. Prescott CA. Protocol for management of the interposition cartilage graft laryngotracheoplasty. Ann Otol Rhinol Laryngol 1988;97:239-42.
7. Morfitt H, Neerken aj, Prevedel A, Liddle eb, Kircher l. Sleeve resections of the trachea. Arch Surg 1955;70:654-61.
8. Grillo hc. Experimental reconstruction of cervical trachea after circunferential resection. Surg Gynec Obstet 1966;122:73-77.
9. Bailey bj, Camp fa. Currents concepts in reconstruction of the cervical trachea. Am J Surg 1969;35:153-65.
10. Drettner B, Lindholm ce. Experimental tracheal reconstruction with composite graft from nasal septum. Acta Otolaryngol 1970;70:401-7.
11. Zalzal gh. Cartilage grafts for the treatment of posterior glottic and sugglottis stenosis in children. Ann Otol Rhinol Laringol 1988;97:509-11.
12. Ilberg c von. Reconstruction chirurgicalle de la trachée par homogreffe de trachée conservée dans du cialit. Paris: Ann Oto-Laryng 1982;99:505-508.
13. Tala P, Maamies tj. Observations in tracheal reconstruction in experimental animals. Annales Chirurgial et Gynaecologicae Fenn 1968;57: 493-6.
14. Atamanyuk my, Melrose dg. The treatment of the cincunferential defects of the tracheas. Brit J Surg 1965;52:59-65.
15. Denecke hj. Reconstruction of the trachea: surgical treatment with flaps and grafts. J Fr Oto-Rhino-Laryngol 1974;23:407-8.
16. Matsumura K, Shors ec, Cohen ah, Jensen ti, Benfield jr. Experimental canine tracheal grafts with reversible squamous metaplasia. Am Rev Respir Dis 1977;116:957-61.
17. Spain dm The distinction between regenerative and atypical alterations in the bronchial mucosa. Am Rev Tuberc 1959;79:591-6.
18. Idriss FA, Deleon SY, Ilbami MN, Gerson CR, Tucker GF, Holinger L. Tracheoplasty with pericardial patch for extensive tracheal stenosis in infants and children. J Thorac Cardiovasc Surg 1984;88:527-36.
19. Anand vk, Alemar g, Warren et. Surgical considerations in tracheal stenosis. Laryngoscope 1992;102:237-243.
20. Ferguson DJ, Wild JJ, Wangensteen OH. Experimental resection of the trachea. Surgery 1950;28:597-619.
21. Davies og, Edmiston m, mccorckle h.J. The repair of experimental tracheal defects with fresh and preserved homologous tracheal grafts. J Thorac Surg 1952;23:367-368.
22. Farrington wt, Hung wc, Binns pm. Experimental tracheal homografting. J Laryng 1977;91:101-10.
23. Alonso wa, Bridger pg, Bordley je. Tracheal transplantation in dogs. Laryngoscope 1972;82:204-9.
24. Seid AB, Pranski SM, Kearns DB. One-stage laryngotracheoplasty. Arch Otolaryngol Head Neck Surg 1991;117:408-10.
25. Wallace MJ, Charnsangavy C, Ogawa K et al. Tracheobronchial tree: expandable metallic stents used in experimental and clinical applications. Work in progress. Radiology 1986;158:309-12.
26. Trachbihler H, Hoelzl J, Dietz FG. Tissue compatibility and biodegradation of new absorbable stents for tracheal stabilization: na experimental study. J Pediatr Surg 1997;32:717-720.
27. Correl no jr, Beattie ej jr. The characteristics of regeneration of respiratory epithelium. Surg Gynec Obstet 1956;103:209-11.
28. Greenberg sd, Willms rk. Regeneration of respiratory epithelium: an experimental study in dogs. Arch Path 1962;65-70.
29. Pearson SE, Stelow EB, Rimell F, Pernell K. Tracheal reconstruction with a synthetic material in a porcine model. Ann Otol Rhinol Laryngol 2001;110:718-722.
30. Greenberg sd. Tracheal homografts in dogs. Arch Otolaryng 1958;67:577-86.
1Full Professor in Otorhinolaryngology, Department of Ophthalmology,
Otorhinolaryngology and Head and Neck Surgery, Medical School of Botucatu - UNESP.
2Assistant Professor, Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Medical School of Botucatu - UNESP.
Endereço para Correspondência: Prof. Dr. Jair Cortez Montovani - Faculdade de Medicina de Botucatu -
Universidade Estadual Paulista "Júlio de Mesquita Filho" UNESP 18.618-0000.
Fax (0xx14) 6822-0421
Article submitted on February 07, 2002. Article accepted on March 13, 2003.


