INTRODUCTIONHead and neck carcinomas are the sixth most common cancer worldwide (INCA, 2006),1 the oral squamous cell carcinoma (OSCC) is the most frequent malignancy in this region. The prognosis of this entity in the mouth is poor; the local invasion and cervical lymph node metastasis rates are high.2,3
Even with advances in oncological therapy, the five-year survival rate for patients with invasive OSCCs remains low (about 35%). Such morbidity rates may be due to factors such as varied responses to chemotherapy and radiotherapy, late presentation and delayed diagnosis. Although this malignancy has uniform morphological features, its biological behavior is heterogeneous, and tumors similarly staged may respond differently to the same treatment.4 Some authors have associated this clinical behavior with clinical staging (TNM) and anatomical site; those located in the tongue and floor of the mouth are considered more aggressive.5 Neck lymph node metastases and the TNM are the main measures of tumor aggressiveness, and are important parameters for planning the approach to such cases.
The metastatic process is complex; cells become dislodged from the primary tumor, the basement membrane and extracellular matrix degrade and are invaded, the tumor cells avoid the immune response in the blood circulation and eventually colonize distant sites.6 This process is how cancer progresses, and is frequent in OSCCs, especially those in the tongue and floor of the mouth, which explains the poor survival rate of patients with this disease.7
Although various metastasis-related events and proteins are known, certain aspects are poorly understood, such as why come tumors metastasize more frequently than others, as well as the identity of genes involved in this process. Identifying these genes may lead to the development of new strategies for the diagnosis and treatment of human cancers.
The nm23 gene appears to be involved in suppressing the metastatic process. It was first described by Steeg et al. in 1988, in experiments where this gene was transfected to mice melanomas. This gene is a family consisting of two genes in mice (nm23-1 and nm23-2) and six genes in humans, which code their respective proteins.6,8 Decreased expression of its protein (nm23) in certain studies has been correlated with an increased metastasis rate and a poor prognosis in patients with breast, ovary, liver, stomach carcinomas and melanomas. This ratio, however, has not been demonstrated in neuroblastomas and bladder carcinomas.9 These findings remain unclear in OSCCs, as the few studies in this area have shown conflicting results.
More recently, a number of studies have investigated the relation between these genes and their expressed proteins with tumors in various anatomical sites; among these studies are those of Bertheau et al.,10 Fishman et al.,11 Kodera et al.,12 and Tokunaga et al.13 These studies have shown that nm23 protein expression is related with metastasis suppression in breast, thyroid, stomach and prostate carcinomas. Gunduz et al.11 found that the same applies to laryngeal squamous cell carcinomas; these authors suggested that the expression of this protein could be used as an indicator of the prognosis.
Improved therapy for OSCC aiming at reducing its metastatic potential and aggressiveness is necessary to increase the survival rate of patients with this disease. The purpose of this paper was to assess the immunohistochemical expression of the nm23-h1 protein in metastatic and non-metastatic squamous cell carcinomas of the tongue.
MATERIAL AND METHODThis retrospective study (a historical cohort) involved selecting 35 cases of squamous cell carcinomas of the tongue. Paraffin-included surgical specimens of patients from which the tumor had been resected and which had or had not done postoperative chemotherapy and/or radiotherapy were obtained. Sections 5 ?m thick were made from these sections, which were then hematoxyllin-eosin stained for morphological analysis.
Cases with at least a 5-year follow-up were selected to investigate the presence of cervical lymph node metastases. Evidence for metastases in these cases was confirmed by image studies, such as computed tomography or magnetic resonance imaging, and/or morphological evidence in cervical dissection.
Sections 3 µm thick were made from the paraffin-included specimens, which were then streptavidin-biotin stained for immunohistochemistry; the sequence was as follows: deparaffinization; hydration in decreasing ethanol sequences; removal of the formolic pigment with 10% ammonium hydroxide in 95º ethanol; blocking of endogenous peroxidase by a 10 volume hydrogen peroxide solution (two 10' steps), followed by antigen recovery in a 25' steamer with the TRIS-EDTA solution (pH 9.0) and incubation of the anti-nm23-h1 monoclonal antibody, at 1:100 dilution, for a 60' incubation time. The material was immersed in a TRIS pH 7.4 buffer solution between reaction steps. Next, it was incubated with a secondary antibody and the streptavidin-biotin complex during 30 minutes at room temperature; the reaction was developed with diaminobenzidine, followed by staining with Mayer's hematoxyllin and mounting on a Permount slide.
Breast ductal carcinoma sections were used as positive controls for verifying the effectiveness of the technique; incubation with the primary antibody was omitted in the negative control.
Two examiners in a double-blind study did the analysis of immune positivity; cases with brownish cytoplasmatic or nuclear staining were considered positive.
The Research Ethics Committee accepted this study (document number 72/03).
RESULTSThe 35 cases of squamous cell carcinomas of the tongue consisted of 19 male cases (54.28%) and 16 female cases (45.71%); the mean age was 65 years. There were 22 patients that smoked cigarettes. Cervical lymph node metastases were found in 15 cases.
The tumor morphology was described as squamous cells with cellular and nuclear pleomorphism, nuclear hyperchromatism, prominent nucleoli, loss of the nuclear/cytoplasmatic ratio, typical and atypical mitoses, nests of varied sizes permeating loose or dense stromal connective tissue, and an inflammatory infiltrate with blood vessels.
Immunohistochemically positive cells were those with a brownish color in the cytoplasm or nucleus (Figure 1) based on the aforementioned analysis.
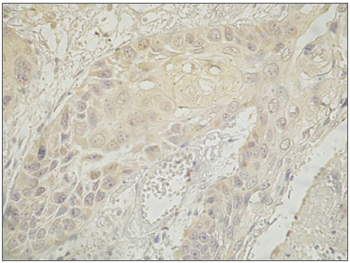
Figure 1. Immunohistochemical expression of the nm23-h1 protein in the cytoplasm of cells in the neoplastic squamous cell carcinoma of the tongue.
Table 1 shows the results of the immunohistochemical analysis of the nm23 protein in our sample of squamous cell carcinomas of the tongue.
Fischer's exact test revealed no statistically significant difference (p=0.365) in the comparison of immunohistochemical nm23 protein marking in metastatic and non-metastatic squamous cell carcinomas of the tongue. Table 1 also shows that there was no positivity in 10 of 15 metastasized cases.
DISCUSSIONAccording to De La Rosa et al.,15 the expression of nm23 gene coded proteins may be verified by using anti-nm23 antibodies, as was done in this study. We used the anti-nm23-h1 antibody in 35 cases of squamous cell carcinomas of the tongue to check whether protein expression occurred in cases of squamous cell carcinomas of the tongue with no metastases to regional lymph nodes. Those authors found that mRNA levels for both the nm23-h1 and the nm23-h2 were decreased in the MDA-MB435 metastatic cell line, compared to the non-metastatic line.
The option to specifically verify the immune expression of the nm23-h1 protein was based on Garzia et al.'s16 statement that the nm23-h1 gene was the first gene to be discovered as having an important role in suppressing metastases. This expression induces anchorage-dependent cell colonization, making it difficult for ells to migrate.
The molecular mechanism of non-metastatic modulation and well-differentiated nm23 phenotypes is poorly understood. Lombardi et al.17 and Postel et al.18 have stated that the nm23 gene and its proteins are expressed physiologically during cell growth and differentiation, and that this expression varies in tissues. It may be found in the nucleus and the cytoplasm, associated with microtubules, on the cell and/or mitochondrial surface. Immune marking in our study was evident both in the nucleus and the cytoplasm of neoplastic cells. Khan et al.7 suggested a further mechanism in which cell migration may be inhibited in OSCCs, even though metalloproteinase 2 and 9 levels remain unaltered.
Ohtsuki et al.19 undertook an immunohistochemical analysis of nm23 protein expression in paraffin-included specimens of 33 OSCC cases and found that there may be suppression of metastases in this condition. Song et al.20 also reached this conclusion in a study of head and neck squamous cell carcinomas. These authors found no correlation with protein expression and the survival rate of patients. Wang et al.21 studied 86 OSCC cases and showed that smoking alters the expression of nm23-h1, facilitating disease progression. This may also have occurred in our sample, as most of our cases were chronic cigarette smokers.
There are few published papers in the literature demonstrating the role of the nm23 protein in suppressing metastases in the mouth. We were motivated to undertake the current study because we found no paper in the world literature that used the nm23 protein only in squamous cell carcinomas of the tongue; most of the published studies had investigated adenocarcinomas.
Fischer's exact test revealed no statistical significance in the expression of the nm23-h1 protein between the metastatic and non-metastatic cases of squamous cell carcinomas of the tongue in our sample. As Table 1 shows, diffuse marking of the nm23-hq protein was more evident in cases with no metastases. A further point is that of 15 metastatic cases, 10 were not immune-positive. Our results are similar to those of Göhring et al.22 in their 2002 paper, where nm23-h1 protein positivity was unrelated to metastatic breast carcinomas. On the other hand, our results disagree with those of Nascimento et al.,23 who investigated benign and malignant salivary gland neoplasms and found that the presence of the nm23 protein in the nucleus may be a good indicator for predicting the metastatic potential of salivary gland malignancies.
CONCLUSIONAlthough we found no statistical significance between squamous cell carcinomas of the tongue and expression of the nm23 protein, we cannot discard its protective role against metastases. Thus, further prospective studies with more uniform clinically staged groups of OCSS cases are needed to demonstrate the aforesaid role in the metastatic process.
REFERENCES1. Instituto Nacional do Câncer. [Site na internet]. Disponível em: http://www.inca.gov.br. Acessado em 14 de dezembro de 2006.
2. Garzino-Demo P, DellAcqua A, Dalmasso P, Fasolis M, La Terra Maggiore GM, Ramieri G.et al. Clinico-pathological parameters and outcome of 245 patients operated for oral squamous cell carcinoma. J Craniomaxillofac Surg 2006;34(6):344-50.
3. Sasaki T, Moles DR, Imai Y, Speight PM. Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J Oral Pathol Med 2005;34(3):129-33.
4. Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol and Endod 2006;102(1):67-76.
5. Costa ALL, Araújo-Júnior R, Ramos CCF. Correlação entre a classificação clínica TNM e as características histológicas de malignidade do carcinoma epidermóide oral. Rev Bras Otorrinolaringol 2005;71(2):181-7.
6. Macdonald NJ, De La Rosa A, Steeg PS. The potential roles of nm23 in cancer metastasis and cellular differentiation. Eur J Cancer 1995;31A(7-8):1096-100.
7. Khan MH, Yasuda M, Higashino F, Haque S, Kohgo T, Nakamura M. et al.. nm23-H1 suppresses invasion of oral squamous cell carcinoma-derived cell lines without modifying matrix metalloproteinase-2 and matrix metalloproteinase-9 expression. Am J Pathol 2001;158(5):1785-91.
8. Gunduz M, Ayhan A, Gullu I, Onerci M, Hosal AS, Gursel B, et al. nm23 Protein expression in larynx cancer and the relationship with metastasis. Eur J Cancer 1997;33(14):2338-41.
9. Wang CS, Lin KH, Hsu YC, Hsueh S. Distant metastasis of gastric cancer is associated with elevated expression of the antimetastatic nm23 gene. Cancer Lett 1998;128(1):23-9.
10. Bertheau P, De La Rosa A, Steeg PS, Merino MJ. NM23 protein in neoplastic and nonneoplastic thyroid tissues. Am J Pathol 1994;145(1):26-32.
11. Fishman JR, Gumerlock PH, Meyers FJ, deVere White RW. Quantitation of NM23 expression in human prostate tissues. J Urol 1994;152(1):202-7.
12. Kodera Y, Isobe K, Yamauchi M, Kondoh K, Kimura N, Akiyama S, et al. Expression of nm23 H-1 RNA levels in human gastric cancer tissues. A negative correlation with nodal metastasis. Cancer 1994;73(2):259-65.
13. Tokunaga Y, Urano T, Furukawa K, Kondo H, Kanematsu T, Shiku H. Reduced expression of nm23-H1, but not of nm23-H2, is concordant with the frequency of lymph-node metastasis of human breast cancer. Int J Cancer 1993;55(1):66-71.
14. Terasaki-Fukuzawa Y, Kijima H, Suto A, Takeshita T, Iezumi K, Sato S. Decreased nm23 expression, but not Ki-67 labeling index, is significantly correlated with lymph node metastasis of breast invasive ductal carcinoma. Int J Mol Med. 2002;9(1):25-9.
15. De La Rosa A, Williams RL, Steeg OS. Nm23/nucleoside diphosphate kinase: Toward a structural and biochemical understanding of its biological functions. BioEssays 1995;17(1):53-62.
16. Garzia L, Roma C, Tata N, Pagnozzi D, Pucci P, Zollo M. H-prune-nm23-H1 protein complex and correlation to pathways in cancer metastasis. J Bioenerg Biomembr 2006;38(3-4):205-13.
17. Lombardi D, Lacombe ML, Paggi, MG. nm23: unraveling its biological function in cell differentiation. J Cell Physiol 2000;182(2):144-9.
18. Postel EH, Abramczyk BM, Levit MN, Kyin S. Catalysis of DNA cleavage and nucleoside triphosphate synthesis by NM23-H2/NDP kinase share an active site that implies a DNA repair function. Proc Acad Sci USA 2000;97(26):14194-7.
19. Ohtsuki K, Shintani S, Kimura N, Matsumura T. Immunohistochemical study on the nm23 gene produce (NDP kinase) in oral squamous cell carcinoma. Oral Oncol 1997;33(4):237-9.
20. Song AU, Mais DD, Groo S, Wright JR, Yoshida GY. Expression of nm23 antimetastatic gene product in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2000;122(1):96-9.
21. Wang YF, Chow KC, Chang SY, Chiu JH, Tai SK, Li WY et al. Prognostic significance of nm23-H1 expression in oral squamous cell carcinoma. Br J Cancer 2004;90(11):2186-93.
22. Gohring UJ, Eustermann I, Becker M, Neuhaus W, Rein DT, Schondorf T. Lack of prognostic significance of nm23 expression in human primary breast cancer. Oncol Rep 2002;9(6):1205-8.
23. do Nascimento KC, de Faria PR, Dib LL, Ferreira de Aguiar MC, Cardoso SV, Chen J, Loyola AM. Immunohistochemical localization of the NM23 protein in salivary gland neoplasms with distinct biological behavior. Virchows Arch 2006;449(6):660-6.
1 Doctorate in oral pathology, UFRN. Adjunct professor of the Universidade do Estado Rio Grande do Norte.
2 Doctorate in oral pathology, UFRN. Adjunct professor, Dentistry Department, Universidade Estadual de Feira de Santana.
3 Master's degree in oral pathology; doctoral student in Health Sciences, UFRN. Responsible for the Dentristry Unit of the Dr. Luiz Antonio Hospital.
4 Doctorate in oral pathology, USP. Adjunct professor of oral pathology, UFRN, and of the graduate program in oral pathology, Universidade Federal do Rio Grande do Norte, Natal/RN.
5 Doctorate in oral pathology, USP. Adjunct professor of oral pathology, UFRN, and of the graduate program in oral pathology, Universidade Federal do Rio Grande do Norte, Natal/RN.
Universidade Federal do Rio Grande do Norte.
Address for correspondence: Lélia Maria Guedes Queiroz - Universidade Federal do Rio Grande do Norte Departamento de Odontologia Programa de Pos-Graduacao em Patologia Oral - Av. Senador Salgado Filho 1787 Lagoa Nova Natal RN 59056-000. Fone/fax: (84) 3215-4138 - E-mail: lmgqueiroz@hotmail.com
This paper was submitted to the RBORL-SGP (Publishing Manager System) on 9 February 2007. code 3657.
The article was accepted on 14 July 2007.


