INTRODUCTIONDeep neck infections (DNI) are bacterial infections originating from the upper aerodigestive tract and involving the deep neck spaces. Although uncommon, these infections are severe, and if not treated adequately, may lead to death. The incidence of this disease was relatively high before the advent of antibiotics, requiring prompt recognition and early interventions.1 Antibiotics resulted in a significant decrease in the occurrence and the progression of this disease. However, when not diagnosed and treated appropriately, these infections progress rapidly and are associated with high morbidity and mortality.
Descending necrotizing mediastinitis is the most feared complication; it results from retropharyngeal extension of infection into the posterior mediastinum. Septic shock is associated with a 40-50% mortality rate.2,3 Pleural and pericardic effusion may accompany this condition, frequently leading to cardiac tamponade. Furthermore, suppurative thrombophlebitis of the internal jugular vein associated with pulmonary septic embolism, thrombosis of the cavernous sinus and erosion of the carotid artery have been reported.4
The purpose of this paper was to retrospectively analyze 80 cases of deep neck infection treated between June 1997 and June 2003. An algorithm for managing this condition is proposed.
MATERIAL AND METHODThe study was a retrospective analysis of 80 treated cases admitted into the emergency unit between June 1997 and June 2003.
Data collection involved demography (age, sex and race), social habits (smoking and consumption of alcoholic beverages), and information on associated diseases.
Also studied were the clinical presentation of the disease, the duration of hospital stay, laboratory exams, the etiology, bacteriological studies, the treatment and the complications.
Computed tomography of the neck and thorax and surgical reports were used for establishing which of the neck spaces were involved by infection.
Also analyzed were the mortality rate and associated factors.
Data were tabulated for descriptive and statistical analysis using the Mann-Whitney test. The SPSS © version 10.0 software was used.
The Research Ethics Committee approved the project (protocol number 224/05).
RESULTSThere were 55 male patients and 25 female patients (Figure 1). The predominant race was white (66 patients), followed by black (7 patients) and brown (7 patients). Fourteen patients had arterial hypertension (17.5%), 19 were diabetic (23.75%), 9 were cardiopaths (11.25%), 4 had lung diseases (5%), 7 had malignant neoplasms (8.75%), 3 were HIV-positive (3.75%) and 3 were users of illegal drugs (3.75%). The mean age was 37.1 years (ranging from 2 months to 94 years) (Figure 2). Smoking was reported by 23 patients (28.75%) and use of alcoholic beverages was reported by 13 patients (16.25%) (Figure 3).
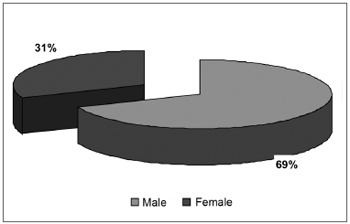
Figure1. Distribution according to gender.
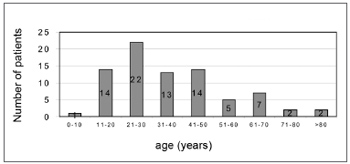
Figure2. Distribution according to age.
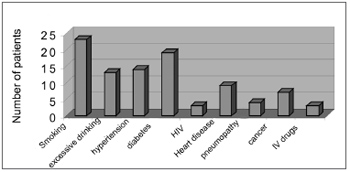
Figure3. Habits and associated disorders.
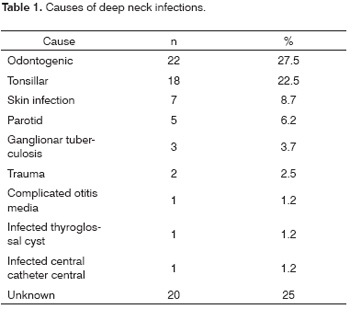
Odontogenic conditions were the most frequent causes of DNI (27.5%), followed by tonsillar diseases (22.5%), skin infection (8.75%) and parotid infection (6.25%). The cause was unclear in 20 patients (25%). Other causes (10%) were ganglionar tuberculosis with abscess (n=3), local trauma (n=2), complicated otitis media (n=1), infected thyroglossal cyst (n=1) and deep infection related to a central venous catheter (n=1). (Table 1)
The symptoms of DNI were neck and/or facial edema in all patients, local pain in 79 patients (98.75%), fever in 68 patients (85%), odynophagia in 19 patients (23.75%), dysphagia in 9 patients (11.25%), difficult breathing in 8 patients (10%) and dental pain in 3 patients (3.75%). The physical examination demonstrated edema of the neck in all patients. There was dental infection in 22 patients (27.5%), peritonsillar infection in 18 patients (22.5%), trismus in 27 patients (33.75%), tachycardia in 20 patients (25%) and toxemia in 19 patients (23.75%). Septic shock was seen in 7 patients (8.75%). A pleural effusion was found in 6 patients (7.5%). Signs of skin necrosis were present in 1 patient. There was hyperemia in the furculum and thorax in 11 patients (13.75%) (Figure 4).
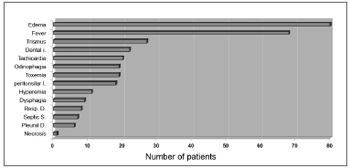
Figure 4. Clinical presentation of deep neck-facial disorders.
The mean progression time of DNI was 8.51 days; the mean hospital stay was 13.3 days.
Airways that were difficult to access occurred in 20 patients (25%); 12 of these patients required endoscopy for intubation, 3 underwent cricothyroidotomy upon admission, and 5 were intubated but considered difficult cases by anesthesiologists. There were no retrograde intubation cases.
Surgery was done in 78 patients, of which 55 patients underwent neck drainage only. Drainage of the neck with debridement of tissues was done in 7 patients; drainage of the neck and tracheostomy was done in 6 patients; drainage of the neck and thoracotomy was done in 5 patients; drainage of the neck and thoracic drainage was done in 4 patients; drainage of the neck and mastoidectomy was done in 1 patient. All of the patients that were drained received endovenous antibiotics. Conservative therapy with endovenous antibiotics and anti-inflammatory drugs was used in 2 patients (Table 2).
Surgical findings were: pus in 64 patients, necrotizing fasciitis with pus in 9 patients, necrotizing fasciitis with no pus in 3 patients, and fasciitis with no necrosis in 2 patients.
The mean white blood count was 16,656 cells per cubic millimeter, ranging from 1,100 to 51,500 cells per cubic millimeter.
Infection was located in the following neck spaces: the submandibulary space in 36 patients, the parapharyngeal and submandibulary spaces in 13 patients, the parapharyngeal space only in 15 patients, the posterior region of the neck in 5 patients, the parapharyngeal, mediastinal and pleural spaces in 5 patients, the parotid space in 2 patients, the retropharyngeal space in 1 patient, the retropharyngeal and mediastinal spaces in 1 patient, the parapharyngeal and mediastinal spaces in 1 patient, and the mastoid region and submandibulary space in 1 patient (Figure 5).
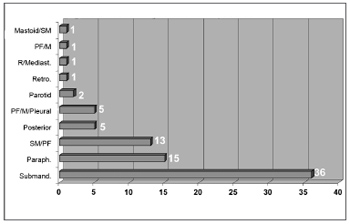
Figure 5. Neck-facial infection sites
The infectious bacteria are shown on Table 3. There were 65 positive cultures. The most commonly isolated bacteria was Staphylococcus aureus in 30 patients (37.5%), followed by Group G Streptococci in 20 patients (25%). Thirteen cultures had no bacterial growth after 48 hours incubation.
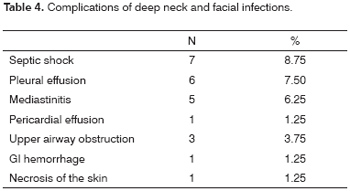
Ten patients had complications, shown on Table 4. Mediastinitis was the most severe complication. Only one of five patients with mediastinitis survived.
The mortality rate was 11.25% (n=9). Of the 9 deaths, 5 were male and 4 were female. Four were diabetics, 1 had bone marrow aplasia, 1 had cancer of the colon that was being treated by chemotherapy, and 1 had gastric cancer. The most common primary cause in these cases was tonsillar disease (n=4), followed by dental conditions (n=3); the origin was not defined in 2 cases. The sites of infection in these cases were: the parapharyngeal space in 8 patients and the retropharyngeal space in 1 patient. The upper mediastinum was involved in 3 of these cases, and the whole mediastinum was involved in 2 of these cases; in the latter there was unilateral pleural effusion. The patient that had involvement of the retropharyngeal space also had a large bilateral pleural effusion. Three patients were reoperated, requiring a second drainage of the neck (n=1) and thoracotomy (n=2). Seven patients died due to septicemia, 1 due to an acute myocardial infarction, and 1 due to a malfunctioning heart pacemaker.
The Mann-Whitney method was used for the statistical analysis to establish possible predisposing factors for a poor prognosis of this infection. The group that progressed to death (demise group) was compared with the group with a favorable outcome (non-demise group).
Clinical presentation, infection site, surgical findings and complication factors, as described above, were analyzed, taking into account a statistical significance of p<0.05.
In the clinical presentation, the presence of tachycardia (heart rate over 80 beat per minute), signs of toxemia, signs of tissue necrosis and respiratory failure were statistically significant factors in the demise group (Table 5).
As to the infection site, involvement of the parapharyngeal space, the association of the parapharyngeal-mediastinal-pleural spaces, or the retropharynx-mediastinum was statistically significant in the demise group (Table 6).
In the surgical findings, only the presence of the association between fasciitis and pus was statistically significant in the demise group (Table 7).
Among the complications, the presence of septic shock and mediastinitis were statistically significant variables associated with the demise group (Table 8).
DISCUSSIONOur results show interesting similarities and differences with the literature.
The mean age that was affected most by infection in the literature varied from 36 to 57 years,2,3,5-10 which was similar to our findings. The disease was twice more frequent in males, as many authors have reported.5-8,11
Smoking and drinking alcohol were the most commonly associated social habits. The most commonly found systemic diseases were diabetes and systemic arterial hypertension. The literature has reported a 16% to 20% incidence of diabetes.8 Systemic arterial hypertension, which may be associated with heart and lung diseases, is not given importance; these factors may have an influence on the morbidity and mortality of DNI. The HIV has been found in 7% of cases6 in the literature; in our study, however, it was found in only 3 patients (3.75%).
The clinical picture of infection with edema of the neck, odynophagia, fever, trismus, a poor health status, associated or not with a primary condition is similar to that found in the literature.8,12 In our study, the presence of tachycardia, toxemia, signs of tissue necrosis and respiratory failure were associated with a poor prognosis in these patients.
DNI originate from a variety of sites in the head and neck; these include the teeth, the salivary glands, lymphoid tissues and the tonsils. The teeth are the most common primary site (31% to 80%), followed by the tonsils (1.5% to 3.4%);1,5-8,10-18 the latter is more frequent in children.19-22 Odontogenic conditions were the most common cause in our series (27.5%), followed by tonsillar disease (22.5%). The cause remained unknown in 20 patients (25%), notwithstanding a detailed clinical history, physical examination and radiological studies. The oropharynx was probably the site of origin in these cases. Other studies have also shown a significant proportion (around 16% to 39%) of DNI of unknown origin.6-9
About two thirds of the cultures of secretions were polymicrobial.6 The most commonly isolated organisms are mostly part of the normal oropharyngeal flora.7 In our series, Staphylococcus aureus was the most commonly isolated bacteria (37.5%), followed by Group G Streptococci (25%). Authors have presented a wide variation of bacteria associated with mixed infection; the most common bacteria that have been encountered have been Streptococci viridans, Staphylococcus epidermidis and Staphylococcus aureus.4,6,8 There was no bacterial growth in 13 cultures (16.25%); this is a low rate compared to that of other authors (which has ranged from 27% to 40%).8,11 This is probably due to the indiscriminate use of antibiotics prior to hospital admittance and the high doses of endovenous antibiotics before surgery.8
The most commonly involved areas in our study were the submandibulary and parapharyngeal spaces. Moncada et al.23 have established the dissemination routes for odontogenic neck infections; these authors demonstrated the anatomical relations between the submandibulary and parapharyngeal spaces, and have explained the pathophysiology of Ludwig's angina. Involvement of the parapharyngeal space, the association between the parapharynx + mediastinum + pleura, or the retropharynx + mediastinum were associated with a poor prognosis.
Since the 1970s, computed tomography has evidently helped improve the diagnosis of DNI.24-26 Tomography of the neck and thorax establish the extension of infection and make it possible to precisely plan the treatment.
All of the patients in whom the abovementioned spaces were involved were treated surgically, except for 2 patients that had a well-defined peritonsillar infection in the suprahyoid area of the neck. These two patients had been using non-prescribed antibiotics for a prolonged period and had no signs of significant toxemia; they progressed well with high doses of endovenous antibiotics, and there was spontaneous intraoral drainage.
Special attention should be given to airway management when patients present trismus or signs of upper airway obstruction, particularly in Ludwig's angina, in which there is edema of the oral floor due to bilateral submandibulary space infection. Parhiscar et al.6 analyzed 210 patients with neck abscesses and reported a need for tracheostomy under anesthesia in 44% of cases, which demonstrates the severity of this condition. We agree that adequate management of airways with intubation using flexible fibroscopy and/or a tracheostomy in cases of significant trismus and edema of the tongue is a priority in the initial approach to DNI.27,28
Our complication rate was 12.5%, which is similar to findings in the literature; these have ranged from 12.85% to 25.5%,6,9,11 mostly associated with mortality. DNI are severe conditions that may rapidly progress to necrotizing fasciitis; descending mediastinitis, in which the mortality rate is 40% to 50%, may or may not complicate the condition, due to its rapid progression to septic shock. The mortality rate in our series was 11.25% (n=9); 7 patients died due to infection and 2 patients died due to non-infectious causes. Mediastinitis and septic shock were related to a poor prognosis of the infection.
Prompt recognition and treatment of DNI are essential for an improved prognosis. Thus, key elements for improved results are the identification of morbid factors, signs and symptoms, and computed tomography.
Based on our experience, we elaborated an algorithm (Figure 6) for managing DNI.
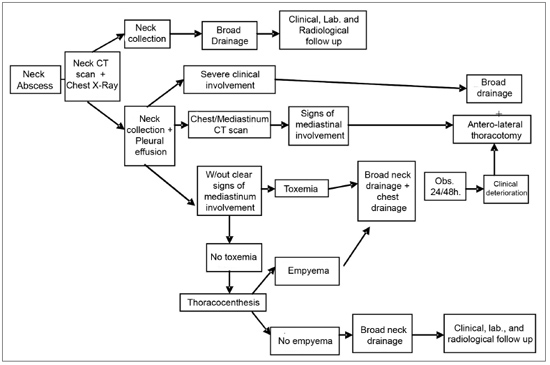
Figure 6. Treatment algorithm in Neck Infections
The current study enabled us to conclude that:
1. Odontogenic and tonsillar causes are the most common;
2. The submandibular and the parapharyngeal areas are the most frequently involved spaces;
3. Staphylococcus aureus and Group G Streptococci are the main microorganisms involved in this condition;
4. A priority in the treatment of DNI should be an adequate management of airways, if surgery is indicated, no muscle relaxant should be given;
5. Surgical drainage is the standard treatment of DNI;
6. Computed tomography is the test of choice for the diagnosis of DNI;
7. DNI have high morbidity and mortality rates, particularly when associated with septic shock and mediastinitis.
8. The proposed clinical management algorithm enables an improved diagnostic and therapeutic approach.
REFERENCES1. Durazzo M, Pinto F, Loures M, Volpi E, Nishio S, Brandão L, et al. Os espaços cervicais profundos e seu interesse nas infecções da região. Rev Ass Med Brasil 1997; 43:119-126.
2. Chen MK, Wen YS, Chang CC, Huang MT, Hsiao HC. Predisposing factors of life-threatening deep neck infection: logistic regression analysis of 214 cases. J Otolaryngol 1998 ;27(3):141-4.
3. Chen MK, Wen YS, Chang CC, Lee HS, Huang MT, Hsiao HC. Deep neck infections in diabetic patients. Am J Otolaryngol 2000;21(3):169-73.
4. Blomquist IK, Bayer AS. Life-threatening deep fascial space infections of the head and neck. Infect Dis Clin North Am 1988;2(1):237-64.
5. Bahu SJ, Shibuya TY, Meleca RJ, Mathog RH, Yoo GH, Stachler RJ, et al. Craniocervical necrotizing fasciitis: an 11-year experience. Otolaryngol Head Neck Surg 2001;125(3):245-52.
6. Parhiscar A, Har-El G. Deep neck abscess: a retrospective review of 210 cases. Ann Oto Rhinol Laryngol 2001;110(11):1051-4.
7. Sakaguchi M, Sato S, Ishiyama T, Katsuno S, Taguchi K. Characterization and management of deep neck infections. Int J Oral Max Surg 1997;26(2):131-4.
8. Sethi DS, Stanley RE. Deep neck abscesses--changing trends. J Laryngol Otol 1994;108(2):138-43.
9. Tom MB, Rice DH. Presentation and management of neck abscess: a retrospective analysis. Laryngoscope 1988;98(8 Pt 1):877-80.
10. Virolainen E, Haapaniemi J, Aitasalo K, Suonpaa J. Deep neck infections. Int J Oral Surg 1979;8(6):407-11.
11. Lin C, Yeh FL, Lin JT, Ma H, Hwang CH, Shen BH, et al. Necrotizing fasciitis of the head and neck: an analysis of 47 cases. Plast Reconstr Surg 2001;107(7):1684-93.
12. Levitt GW. The surgical treatment of deep neck infections. Laryngoscope 1971;81(3):403-11.
13. Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR. Descending necrotizing mediastinitis. Surg Gynecol Obstet 1983;157(6):545-52.
14. Furst IM, Ersil P, Caminiti M. A rare complication of tooth abscess--Ludwigs angina and mediastinitis. J Can Dent Assoc 2001;67(6):324-7.
15. Georgalas C, Kanagalingam J, Zainal A, Ahmed H, Singh A, Patel KS. The association between periodontal disease and peritonsillar infection: a prospective study. Otolaryngol Head Neck Surg 2002;126(1):91-4.
16. Johnson JT, Tucker HM. Recognizing and treating deep neck infection. Postgrad Med 1976;59(6):95-100.
17. Smith L, Osborne R. Infections of the head and neck. Top Emerg Med 2003;25(2):106-16.
18. Umeda M, Minamikawa T, Komatsubara H, Shibuya Y, Yokoo S, Komori T. Necrotizing fasciitis caused by dental infection: a retrospective analysis of 9 cases and a review of the literature. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 2003;95(3):283-90.
19. Choi SS, Vezina LG, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otolaryngol Head Neck Surg 1997;123(12):1271-5.
20. Cmejrek RC, Coticchia JM, Arnold JE. Presentation, diagnosis, and management of deep-neck abscesses in infants. Arch Otolaryngol Head Neck Surg 2002;128(12):1361-4.
21. Hartmann RW, Jr. Ludwigs angina in children. Am Fam Physician 1999;60(1):109-12.
22. Nicklaus PJ, Kelley PE. Management of deep neck infection. Pediatr Clin North Am 1996;43(6):1277-96.
23. Moncada R, Warpeha R, Pickleman J, Spak M, Cardoso M, Berkow A, et al. Mediastinitis from odontogenic and deep cervical infection. Anatomic pathways of propagation. Chest 1978;73(4):497-500.
24. Endicott JN, Nelson RJ, Saraceno CA. Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography. Laryngoscope 1982;92(6 Pt 1):630-3.
25. Holt GR, McManus K, Newman RK, Potter JL, Tinsley PP. Computed tomography in the diagnosis of deep-neck infections. Arch Otolaryngol 1982;108(11):693-6.
26. Munoz A, Castillo M, Melchor MA, Gutierrez R. Acute neck infections: prospective comparison between CT and MRI in 47 patients. J Comp Ass Tomogr 2001;25(5):733-41.
27. Heindel DJ. Deep neck abscesses in adults: management of a difficult airway. Anesth Analg 1987;66(8):774-6.
28. Shockley WW. Ludwig angina: a review of current airway management. Arch Otolaryngol Head Neck Surg 1999;125(5):600.
1 Head and neck surgeon, assistant in the Head & Neck Surgery Discipline, Sao Paulo Santa Casa.
2 Adjunct professor, chief of the Head & Neck Surgery Discipline, São Paulo Santa Casa.
3 Doctoral degree, assistant in the Head & Neck Surgery Discipline, São Paulo Santa Casa.
4 Master's degree, assistant in the Head & Neck Surgery Discipline, São Paulo Santa Casa.
5 Doctoral degree, assistant in the Head & Neck Surgery Discipline, São Paulo Santa Casa.
Medical Science School, Sao Paulo Santa Casa, Head & Neck Surgery Discipline.
Address for correspondence: Rua Dom Armando Lombardi 471 apto. 33 Jardim Guedala 05616-011 Sao Paulo S.P
Paper submitted to the ABORL-CCF SGP (Management Publications System) on January 27th, 2007 and accepted for publication on March 24th, 2007. cod. 3630.


