INTRODUCTIONIn the last years, larynx electromyography (EMG) has been used in the diagnosis of vocal fold mobility disorders, especially differentiating fixation from paralysis, providing data for prognosis and location of neuromuscular lesions1-10, and configuring an objective method to assess neuromuscular activity3, 11, 12.
Vocal fold paralysis can be the result of central or peripheral lesion that involves the vagus nerve above the emergence of the superior laryngeal nerve (SLN), or more distally, without its involvement, that is to say, impairing only the recurrent nerve (RN). The position taken by the paralyzed vocal fold can vary from medial to lateral, and it determines in part how the vocal folds coaptate and the severity of symptoms. There are frequent discussions about the factors responsible for the vocal fold paralytic position and many studies tried to relate them to the action of the cricothyroid muscle (CT), innervated by the external branch of the SLN 13-15. Although some studies supported the role of CT by the adducting force in the position taken by the vocal folds 14-16, other studies, as well as clinical applications, did not confirm these data17-20. The vocal folds position is not directly related to the cause of the paralysis and various degrees of chinks can be associated with RN paralysis21.
The purpose of the present study was to investigate the functional interaction between the intrinsic laryngeal muscles based on the data collected by laryngoscopy and laryngeal EMG in one case of laterally immobilized vocal fold.
CASE REPORTFemale 48-year old patient, Caucasian, married, born in São Paulo, complaining of sudden onset aphonia for 40 days - she woke up one morning and had the vocal disorder. She did not report other complaints. We conducted complete ENT examination, including telelaryngoscopy after topical oropharynx anesthesia with 10% lidocaine to prevent nausea reflex. Images were recorded during inspiration and production of sustained vowel 'é'. Laryngoscopy showed loss of mobility of the left vocal fold fixed at the lateral position (Photos 1 a, 1b, and 1c), despite the evident swinging movement of the left arytenoid. We conducted EMG of CT, thyroarytenoid (TA) and lateral cricoarytenoid (LCA) muscles. The EMG was performed with the patient lying down and slightly extending the neck, using transcutaneous route and no anesthesia. The muscles were tested in the following sequence 1) CT; 2) TA, 3) LAC, initially on the clinically healthy side, followed by the impaired side. We used the device Nihon-Kohden Sigma, monopolar concentric electrode needle and 10ms speed per division of the motor unit potential recording. The electrical activity of the muscles was visually monitored by the oscilloscope, and by the sounds produced. Representative examples were stored for later analysis and printing in photosensitive paper. The muscles were tested at rest, deep inhalation and phonation for vowel emission ½½ and ½æ½.
CT was tested by introducing the needle at the level of the space between the thyroid and cricoid cartilages, approximately 1cm laterally to the midline and during emission of vowel ½½; we asked the patient to turn the neck and swallow before and after the test to make sure that we did not have the needle on the sternothyroid or sternohyoid muscles7, 22. The TA muscle analysis was transcutaneous through the cricothyroid membrane, with the needle at approximately 45o above and 2cm laterally to the midline. For the positioning of the needle in the LAC, the head was slightly rotated to the opposite side and the needle was introduced approximately 1.5cm from the midline, following the upper border of the cricoid cartilage, up to the muscle.
The study of motor unit potentials included the analysis of recruitment and the wave morphology of each tested muscle, including the insertion activity, voluntary action potentials and the presence of spontaneous activity.
The EMG of tested muscles on the right side was normal (Figures 1, 3 and 5), as well as the muscles CT and TA on the paralyzed side (Figures 2 and 4); the LAC muscle on the same side showed reduced recruitment and capture of recovering potentials (Figure 6). The follow-up showed regression of paralysis 2 months after the EMG, with normal recruitment and recovery of vocal quality and movement of left vocal fold (Photos 2 a, 2b and 2c).
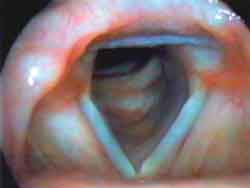
Photo 1a. Laryngoscopy during paralysis: inspiration
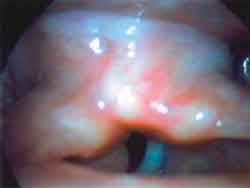
Photo 1b. Laryngoscopy during paralysis: phonation ½ æ ½ ("é")
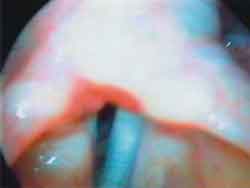
Photo 1c. Laryngoscopy during paralysis: phonation½ ½ ("i"). Note the absence of abnormalities during inspiration and the vocal fold fixed at the lateral position during phonation.
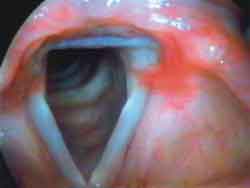
Photo 2a. Laryngoscopy after mobility restore: inspiration
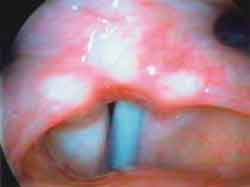
Photo 2b. Laryngoscopy after mobility restore: phonation ½ æ ½ ("é")
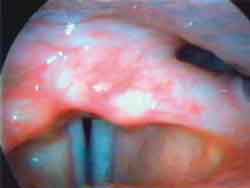
Photo 2c. Laryngoscopy after mobility restore: phonation½ ½ ("i").
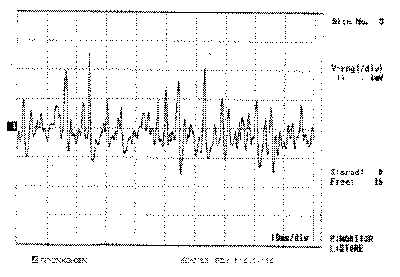
Figure 1. Electromyography of right cricothyroid muscles
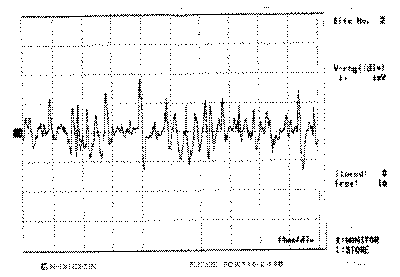
Figure 2: Electromyography of left cricothyroid muscle
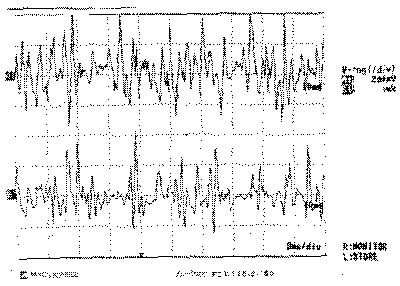
Figure 3. Electromyography of right thyroarytenoid muscle
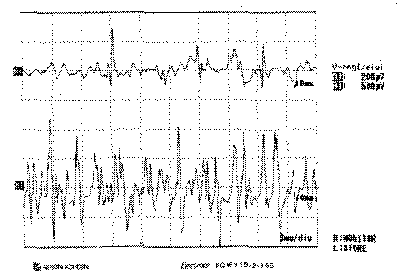
Figure 4. Electromyography of left thyroarytenoid muscle
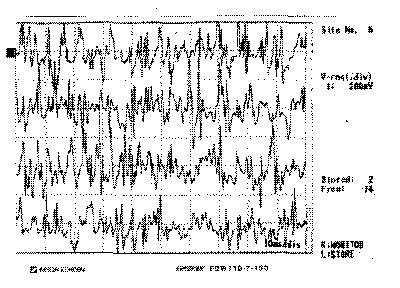
Figure 5. Electromyography of right lateral cricoarytenoid muscle
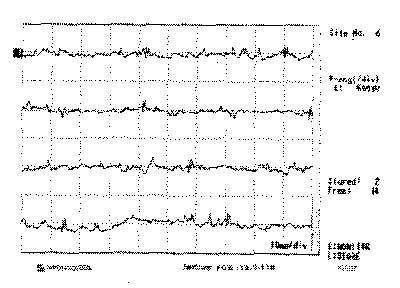
Figure 6. Electromyography of left lateral cricoarytenoid muscle, showing the recovering potentials
Laryngeal nerves are innervated by two branches of the vagus nerve, the RN and SLN. RN is responsible for the motor innervation of all intrinsic ipsilateral laryngeal muscles, except the CT23, 24. Vocal fold immobility can be a consequence of paralysis by loss of innervation or mechanical fixation of the vocal folds. The inferior laryngeal nerve branch innervates successively the posterior cricoarytenoid muscle (PCA), interarytenoid (IA) and the terminal branch that is directed to the LCA and finally to the TA24. Normally, the muscles tested during the EMG are the CT and the TA, and indirectly through them the activity of the superior and inferior laryngeal nerves, respectively11, 24, 25. For Dedo14, all the branches of the intrinsic laryngeal muscles arise from the RN after the penetration into the larynx, protecting the thyroid cartilage from trauma, tumors and neuritis. For this reason he considers that all muscles are affected as a whole if there is RN impairment. In a study with 45 patients with paralysis, the author found TA potentials in 2 cases, considering them the result of nerve irritation, partial dennervation or reinsertion, leading to non-effective contraction. Faaborg-Andersen26 considered possible the paralysis of adductors without lesions of the first branch of the abductor muscle, which would result in paralysis at intermediate position. However, in his opinion, if there is normal function of CT, IA and LCA there should be vocal fold movement, despite not being normal in extension. Thus, the detection of the lateral position with no vocal fold movement (Photos 1 a, 1b and 1c) and preservation of TA activity (Figure 4) would make us wonder whether there was fixation of the cricoarytenoid articulation, another diagnosis to be considered, or the hypothesis that normal activity of TA muscle would not necessarily mean nerve normal status. The isolated lesion impairing only LCA, responsible for the medial rotation of the arytenoid vocal process13, was the other hypothesis considered. The result of the EMG confirmed the validity of this hypothesis (Figure 6).
Sanders et al.24 observed innervation of muscles IA by inferior laryngeal nerves of both sides forming anastomotic plexus between each other and with the SLN branch. The authors suggested that this fact could explain the position of the paralyzed vocal folds, which would be variable depending on the presence of occasional motor neurons of superior laryngeal nerve. Dedo14 disagreed that the weakened contraction of IA would be responsible for the medial position because he observed arytenoid and vestibular folds slight movements in bilateral recurrent paralysis. He considered that the CT muscle would be the main responsible for keeping the paralyzed vocal folds in the medial position in unilateral recurrent paralysis. Woodson20 did not observe statistically significant differences between the angle formed by the vocal folds considering groups of patients with complete lesions only in the recurrent or vagal nerves, concluding that the CT muscle was not a determining factor in the position of the paralyzed larynx. The same author, Hiroto et al.27, Faaborg-Andersen26, Crumley28 and Thumphart12 reported EMG normal activity in patients with vocal fold immobility and suggested that simultaneous contraction of antagonist muscles after syncinesis could result in immobility during phonation. Woodson20 considered the possibility of reinnervation of the adductor intrinsic muscles, with differences in numbers and size of the motor units leading to varied positions, but he also realized that it was unlikely due to the immobility observed, what would imply perfect balance of the opposite forces. In the case reported here, the effective and visible movement of arytenoids by the contraction of IA muscle did not result in abnormality of the position taken by the paralyzed vocal fold (Photos 1 a, 1b 1c). Such fact confirms that the contraction of IA does not medialize the vocal folds. Not even the contraction of CT, in synergism with IA, manages to medialize the vocal folds in phonation or keep them paralyzed in the most medial position. We could assume that in addition to paralysis of LCA muscle, confirmed by EMG, there would be concomitant cricoarytenoid joint ankylosis. The progression, however, contradicts this hypothesis, since the vocal fold restored normal mobility (Photos 2 a, 2b and 2c), already expected by the EMG tracing with recovering potentials that indicated dennervation but also evidenced reinnervation (Figure 6). The isolated paralysis of LCA observed in our study showed the possibility of having only one RN branch affected.
CLOSING REMARKSThe fact of having exclusive dennervation of LCA muscle, with preservation of the other muscles that share adducting functions, shows the importance of this muscle as the main adductor, since the patient's vocal fold remained in the lateral position, even with approximation of the arytenoids, resulting in marked dysphonia. This case of isolated paralysis of LCA muscle provided the understanding of the functional laryngeal anatomy, pointing to a management approach to differentiate recurrent paralysis from fixation. Thus, we believe that in the presence of normal TA muscle response, the other adductors should be tested to reach a precise diagnosis, except IA that has bilateral innervation 24 and always presents normal EMG tracings in unilateral recurrent paralysis27.
REFERENCES 1. Dursun G, Sataloff RT, Spiegel JR, Mandel S, Heuer RJ, Rosen DC. Superior Laryngeal Nerve Paresis and Paralysis. J Voice 1996;10(2): 206-10.
2. Kotby MN, Fadly E, Madkour M, Khidr A, Alloush T, Saleh M. Electromyography and Neurography in Neurolaryngology. J Voice 1992;6(2): 159-87.
3. Kotby N & Haugen LK Clinical application of electromyography in vocal fold mobility disorders. Acta Otolaryngol 1970;70: 426-237.
4. Koufman JA, Walker FO, Joharji GM. The Cricothyroid Muscle Does Not Influence Vocal Fold Position in Laryngeal Paralysis. Laryngoscope 1995;105: 368-72.
5. Min YB, Finnegan EM, Hoffman HT, Luschel ES, McCullock TM. A preliminary study of the prognostic role of electromyography in laryngeal paralysis. Otolaryngol Head Neck Surg 1994;111(6): 770-75.
6. Parnes SM, Satya-Murti S. Predictive value of laryngeal electromyography in patients with vocal cord paralysis of neurogenic origin. Laryngoscope 1985;95:1323-26.
7. Rodriguez AA, Myers BR, Ford CN. Laryngeal Electromyography in the Diagnosis of Laryngeal Nerve Injuries. Arch Phys Med Rehabil 1990;71:587-90.
8. Sataloff RT, Bough Jr D, Spiegel JR. Arytenoid dislocation: Diagnosis and Treatment. Laryngoscope 1994;104: 1353-61.
9. Thumfart WF. From Larynx to Vocal Ability. Acta Otolaryngol 1988 (Stockh) 105: 425-31.
10. Yin SS, Qiu WW, Stucker FJ. Value of electromyography in differential diagnosis of laryngeal joint injury after intubation. Ann Otol Rhinol Laryngol 1996;105: 446-451.
11. Rontal E, Rontal M, Silverman B, Kileny P. The Clinical Differential Between Vocal Cord Paralysis and Vocal Cord Fixation Using Electromyography. Laryngoscope 1993;103:133-7.
12. Thumphart WF. Electrodiagnosis of Laryngeal Nerve Disorders. Ear Nose Throat 1988;67:380-93.
13. Benninger MS, Crumley RL, Ford CN, Gould WJ, Hanson DG, Ossof RH, Sataloff RT. Evaluation and Treatment of the unilateral paralyzed vocal fold. Otolaryngol Head Neck Surg 1994;111:497-508.
14. Dedo HH. The paralyzed larynx: an electromyographic study in dogs and humans. Laryngoscope 80(10):1455-1517.
15. Manon-Espaillat R, Mandel S, Sataloff RT. Laryngeal Electromyography. In: Sataloff RT. Professional Voice: the science and art of clinical care. 2nd. San Diego: Singular Publishing; 1997. p. 245-55.
16. Tucker HM. Neurologic Disorders. In: ____________. The Larynx. 2nd. Ed. New York: Thieme Medical Publishing; 1993. p. 246-65.
17. Cohen S & Thompson TW. Use of Botulinum Toxin to lateralize true vocal cords: A biochemical method to relieve bilateral abductor vocal cords paralysis. Ann Otol Rhinol Laryngol 1987;96:534-541.
18. Woodson GE, Murry M, Schweizer V, Hengesteg A, Chen N, Yeung D. Unilateral Cricothyroid Contraction and Glottic Configuration. J Voice 1998;12(3):335-39.
19. Woodson GE. Configuration of the Glottis in Laryngeal Paralysis. I: Clinical Study. Laryngoscope 1998;103:1227-34.
20. Woodson GE. Configuration of the Glottis in Laryngeal Paralysis. II: Animal Experiments. Laryngoscope 1993;103:1235-41.
21. Koufman, JA. Management of the paralyzed vocal cord. [online]. Available Internet.
[nov 05 1999]
22. Hirano M & Ohala J. Use of Hoocked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res 1969;12:362-373.
23. Garret JD & Larson CR. Neurology of the Laryngeal System. In: Ford C & Bless D. Phonosurgery: assessment and surgical management of voice disorders. New York: Raven Press; 1991. p. 43-75.
24. Sanders I, Wu B, Mu L, LI Y, Biller HF. The Innervation of the Human Larynx. Arch Otolaryngol Head Neck Surg 1993;119:934-9.
25. Simpson DM, Sternman D, Graves-Wright J, Sanders I. Vocal cord Paralysis: clinical and electrophysiologic features. Muscle & Nerve 1993:952-57.
26. Faaborg-Andersen K. The position of paretic vocal cords. Acta Oto-laryng 1964;57:50-4.
27. Hiroto I, Hirano H, Tomita H. Electromyographic investigation of human vocal cord paralysis. Ann Otol Rhinol Laryngol 1968;77:296-304.
28. Crumley RL. Unilateral Laryngeal Nerve Paralysis. J Voice 1994;8(1):78-83.
1 Ph.D. in Medicine, Federal University of São Paulo - Escola Paulista de Medicina (UNIFESP - EPM). Assistant Professor, Ph.D.,
School of Speech Pathology and Audiology, Pontifical Catholic University of São Paulo - PUC-SP.
2 Faculty Professor, Department of Otorhinolaryngology and Human Communication Disorders, Federal University of
São Paulo - Escola Paulista de Medicina (UNIFESP - EPM). Inlar - Instituto da Laringe - São Paulo.
3 Joint Professor, Department of Neurology and Neurosurgery, Federal University of São Paulo - Escola Paulista de Medicina (UNIFESP - EPM). Head of the Service of Electroneurophysiology, Federal University of São Paulo - Escola Paulista de Medicina (UNIFESP - EPM).
4 Undergraduate, Medical School, Pontifical Catholic University of São Paulo.
Study presented at the 2nd. World Voice Congress, in February 1999 in the city of São Paulo.
Address correspondence to: Noemi De Biase - Rua Dr. Diogo de Faria, 171 - Vila Clementino - 04037-000 - São Paulo - Brazil - Tel. (55 11) 5549-2188 - Fax (55 11) 5575-9649 - E-mail: nbiase@terra.com.br
Article submitted on November 23, 2001. Article accepted on November 29, 2001.


