IntroductionIn Brazil, incidence of malignant neoplasias of the oral cavity is substantially varied in different regions and this fact is probably due to local features concerning prevalence of risk factors1. Many risk factors are related to oral squamous cell carcinoma etiology, among which tobacco, betel, alcohol, virus and diet are included. These factors are associated with genetic inheritance and may have a carcinogenic effect on normal cells of respiratory and digestive systems2. Oral squamous cell carcinoma may occur anywhere in the mouth, although the most affected sites are tongue, lower lip and floor of the mouth. Features of these regions greatly facilitate carcinoma spreading to regional lymph nodes and/or distant organs3.
In patients with head and neck carcinoma, the prognosis is usually obtained based on TNM clinical classification, which is highly useful, specially to assess essential features of cancer, such as local extension, regional dissemination and distant metastasis4.
Patient's prognosis and survival5 may be determined with this system, which must include details of local anatomical characteristics to obtain data on grade of tumor involvement, as well as on distant metastases. However, the use of other prognostic markers is fundamental, once it helps to plan treatment and estimate patient's survival, which most of the times is reduced due to local recurrence or early lymph node metastases, added to existence of inaccurate prognostic factors6,7. Histological staging of the deepest parts of oral squamous cell carcinoma directly influences patient's survival, once neoplastic cells in this site appear to be undifferentiated when compared to other tumor regions, constituting a valuable prognostic mean. Histological staging systems are very important, once they highlight the histopathological characteristics and the immunological relationship between tumor and host, predicting lesion's behavior in face of patient's response8,9. Many studies were carried out to confirm correlation between clinical and histological parameters and prognosis. Keratinization grade, nuclear pleomorphism, number of mitoses, invasion mode and lymphatic infiltration were used by many authors as histopathological characteristics studied in superficial areas of tumor11. Byrne, in 1989, using the same histological markers (except for number of mitoses), suggested histological staging in invasive margins of lesion once he considered it to be the most representative region of neoplasia11.
Dib, Sabba (1994)10 have studied the medical records of 59 patients with oral squamous cell carcinoma between the period of 1960 and 1980. Histological and clinical variables such as sex, age, clinical staging (TNM) and presence of palpable lymph nodes were observed. These authors verified that clinical stage I patients presented higher overall survival rate, while those of clinical stage IV had lower survival. Moreover, tendency to keratinization and tumor thickness presented statistically significant correlation with overall survival10. On the other hand, other authors in similar studies - except for the idea that only the most invasive parts were to be graded - concluded that the invasion pattern was the histological staging system that had the most important correlation with clinical parameters, such as metastasis and recurrence7,11.
Therefore, it was verified that this type of staging studies is more complex due to the heterogeneous features between oral squamous cell carcinoma subtypes and different behaviors this tumor has in different areas of oral mucosa. Furthermore, some clinical variables are not taken into account or investigated by the professional that performs the anamnesis of cancer patient. Considering that, this research study aimed at correlating TNM clinical classification with histopathological characteristics (keratinization grade, nuclear pleomorphism, invasion pattern and lymphoplasmocitary infiltrate) and the histological malignancy scores in 38 cases of oral squamous cell carcinoma in deep areas of the lesion, as described by Bryne (1998)9.
Material and MethodsPopulationConsisted of all cases of oral squamous cell carcinoma of Hospital Dr. Luis Antonio in the city of Natal / RN, in the period between 1990 and 1995. After the project was evaluated and approved by the Committee of Ethics of Hospital Universitário Onofre Lopes, the medical records of all patients with oral squamous cell carcinoma were searched.
SampleThe selected sample consisted of 38 patients with oral squamous cell carcinoma assisted and registered in the medical records of Hospital Dr. Luiz Antonio. Records' analysis included information on gender and age of patients, anatomical sites of lesions and clinical TNM classification. These data were registered in a pre-developed simplified clinical filing card. The cases were selected according to the following parameters:
1 Gender and skin color: men and women, considering Caucasians, Mulattos and Black descendents.
2 Age: in age ranges, starting from the 4th. decade
3 Site of lesion: lesion was analyzed according to its localization in the side border of tongue, lower lip, mouth floor, oral pharynx or jugal mucosa.
4 TNM clinical staging: the version established by the International Union Against Cancer was employed (Hermaneck et al.12, 1996; Neville et al.13, 1998): Stage I = T1 N0 M0; Stage II= T2N0M0; Stage III= T3N0M0, or T1, T2 or T3N1M0; Stage IV= any T4 lesion, any N2 or N3 lesion, or any M1 lesion.
Records with incomplete or confused data regarding clinical variables were disregarded and their respective paraffin blocks were not selected for further laboratory procedures.
Morphological analysis and histological malignancy stages of 3µm-thick tumor sections were conducted by two pathologists, using previously gauged optical microscopy and hematoxylin and eosin stain technique (H&E) in the deepest areas of the tumor. The sections were analyzed at the laboratory of Oral Pathology and further under light microscopy at the Department of Oral Pathology, UFRN.
Pre-selected cases of oral squamous cell carcinoma were assessed and classified by the histological malignancy staging system developed by Bryne (1998)9. The study consisted of evaluation of four morphological features: degree of keratinization, nuclear pleomorphism, invasion pattern and lymphocytary infiltrate. Total average scores of histological malignancy staging were obtained by summing the scores attributed to each morphological parameter divided by the number of parameters used, as to reduce occasional distortions at the final count. Therefore, following Bryne's system (1998)9, they considered 1.0-2.5 range as low malignancy score, and 2.6 to 4.0 range as high score (Table 1).
Statistical analysis For results' analysis, following the Gaussian distribution, the parametric analysis was chosen (ANOVA). However, considering that some types are nominal qualitative variables, the non-parametrical Q-square, Tukey and Pearson tests were also applied. A 5% rejection index of null hypothesis was applied to all tests performed.
ResultsBased on data analysis of 38 patients with oral squamous cell carcinoma presented in this study, the sample consisted of: 55.26% (21 cases) of men and 44.75% (17 cases) of women (Figure 1) between the ages of 40 and 93 years. The most prevalent age range was 51 to 60 years, represented by 57% of the cases (12 patients) (Figure 2).
Regarding the anatomical site of the lesion, it was observed that the side border of tongue was the most affected region in 50% of the sample (19 cases), followed by the lower lip - 26.31% (10 cases), and floor of the mouth - 13.15%, (5 cases). Other areas such as the upper lip (2.63%), jugal mucosa (2.63%), oral pharynx (2.63%), and tongue center (2.63%) were also affected, summing 10.52% (4 cases) of the sample (Figure 3).
Concerning TNM clinical staging, 14 patients were classified as stage IV, 11 as stage III, 5 as stage II and 8 as stage I (Figure 4).
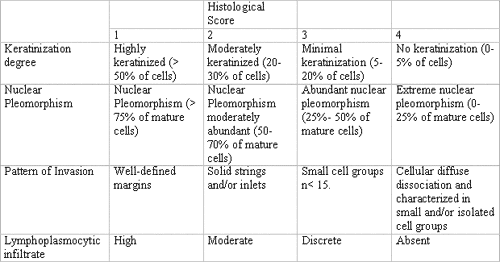
Table 1. Histological malignancy staging system according to Bryne (1998).
As results using Tukey's statistical test were analyzed, a significant correlation was observed among combinations of TNM clinical stages when related to the following histopathological parameters: degree of keratinization (p=0.05) and nuclear pleomorphism (p= 0.02). The same correlation was also verified with the average histological score (p=0.01) (Table 2).
The statistical analysis of variance revealed that the degree of keratinization (p= 0.025), nuclear pleomorphism (p= 0.016) and average malignancy score (p= 0.001) had statistically significant correlation with TNM clinical staging (Table 3). Pearson's test showed correlation between the average histological scores and the isolated variables of TNM clinical staging (p< 0.005) (Table 4).
When TNM clinical stages were grouped into two series - I/II and III/IV - and related with the histopathological variables using the Q-square test, a correlation between these groups and the lymphoplasmocytic infiltrate (p= 0.016) and nuclear pleomorphism (p= 0.004) (Table 5) was also observed.
DiscussionPrognosis of patients with oral squamous cell carcinoma is significantly varied due to different histological types and clinical features of this entity. Analysis of the TNM system and the histological staging has been adopted to plan treatment and provide better evaluation of patient's evolution. Patients with advanced tumors have poor prognosis, in which regional metastasis is the most important prognostic indicator12,13.
Some clinical findings are discussed in this study to elucidate the relation between the events and the aspects approached, as well as to give rise to reflections that may contribute for new investigations.
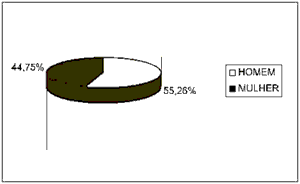
Figure 1. Number of patients distributed by gender.
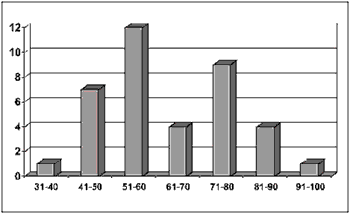
Figure 2. Number of patients distributed by age range.
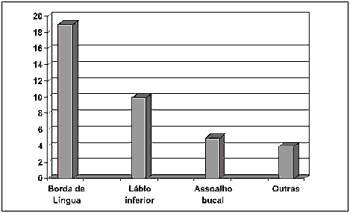
Figure 3. Number of patients distributed by anatomical site.
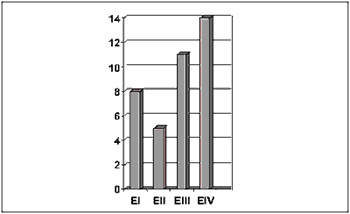
Figure 4. Number of patients distributed by TNM clinical stage.
Regarding clinical data, the sample showed that 55.26% of patients (21 cases) were men and that the cases within the 50-70-age range were the most affected ones (25 cases). No patients under 30 years old were registered, which is consistent with the literature available (Figures 1 and 2) 4,10,14-17.
Concerning anatomical, the side border of tongue (19 cases) followed by the lower lip (10 cases) were the most affected regions, which is in accordance with the data reported by other studies4,18,19 (Figure 3).
TNM clinical classification is one of the most used systems to predict the prognosis of oral squamous cell carcinoma. However, there are some divergences concerning tumor staging. It is a fact when dealing with primary tumors that advanced cases with infiltration of adjacent structures are hardly accessed at clinical examination 20,21. Regardless of the limitation of TNM clinical staging, its accuracy is around 70 to 80%. This percentage is higher when complementary exams, such as computed tomography and magnetic resonance, are used for classification and staging of neoplasias20. It is perfectly feasible to admit that results obtained by histological assessment are more accurate in prognostic settlement than essentially clinical exams, once microscopic findings provide information on events that are previous to those of clinical nature.
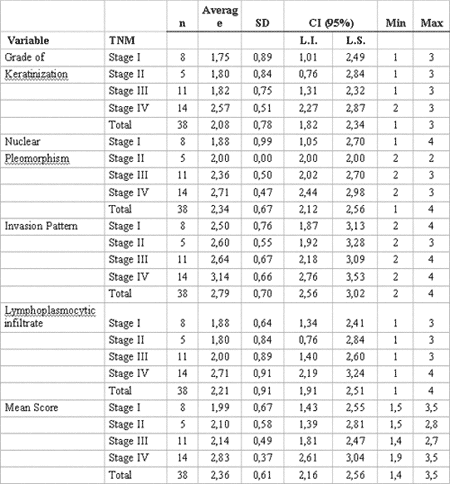
Table 2. Descriptive statistics of variables, according to stage. Natal, RN. 2004.
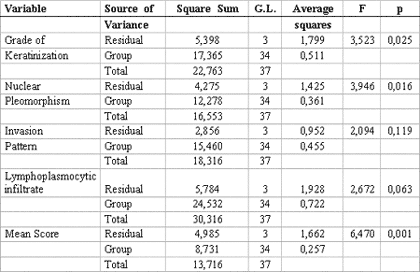
Table 3. Analysis of variance for independent variables in relation to stage. Natal, RN. 2004.
In order to assess an eventual correlation of TNM clinical staging with histological malignancy parameters and the average score proposed in our trial, a descriptive statistical analysis was conducted demonstrating statistically significant correlation between them 4,9,22-24 (Table 2). However, these results changed when a variance analysis of independent histological parameters was performed in relation to the grade of TNM clinical staging, showing that invasion pattern (p= 0.119) was the only parameter that had no significant statistical relation with TNM clinical stage (Table 3).
The statistical analysis by Pearson's correlation test straightens the results of the statistics of variance, once there was correlation between the average histological scores and the clinical variables of TNM clinical staging (p< 0.005) when observed isolated 9,22 (Table 4).
The histological parameter "invasion pattern" is a valuable prognostic factor, once it reflects the capacity of cohesion of neoplastic cells23, suggesting that well-differentiated neoplasias invade within a well-defined margin pattern, while more anaplastic tumors infiltrate as small cell aggregates or isolated cells19. We understand that this explains its correlation with the prognosis.
Regarding TNM clinical stages of 38 cases distributed by I/II and III/IV groups and its relation with histological variables, a significant correlation was observed in these groups with lymphoplasmocytic infiltrate (p= 0.016) and nuclear pleomorphism (p= 0.004). These results may translate the possible effect of TNM clinical staging over tumor histological behavior, once III/IV patients presented poorer histological behavior. Contrary to our findings, Lopes et al. (2002)15 observed that the keratinization degree (p< 00.1) is the best histological parameter to determine the entity's clinical behavior and the risk of developing regional metastasis. In addition, the authors also verified that T1/T2N+ tumors presented high percentage of neoplastic cells with plentiful nuclear pleomorphism (p< 0.05), while T3/T4N0 tumors showed " invasion pattern" histological parameter in small-cell like groups in 65% of the cases (Table 5).
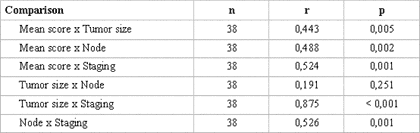
Table 4. Pearson's correlation test between markers and according to type of lesion studied. Natal, RN. 2004.
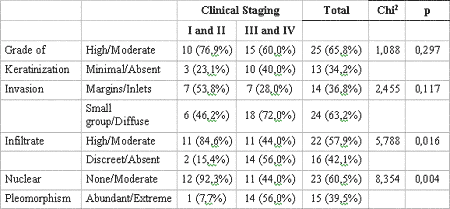
Table 5. Relation between presence of features and clinical staging of lesion. Significance obtained by Chi2 test. Natal, RN, 2004.
Histological staging of the most invasive margin, as suggested by Bryne (1998)9 and adopted in our trial, provides the best prognostic information on the neoplasm. Differently from former systems in which only the superficial part of the tumor was observed25-27, this author provides the pathologist9 with thorough information on anaplastic cells behavior.
Closing remarksBy the end of our study, we concluded that keratinization degree, nuclear pleomorphism and lymphoplasmocytic infiltrate are strongly correlated to TNM clinical staging. The latter is strongly associated with malignancy scores, constituting important prognostic indicator. Therefore, there was statistically significant correlation between clinical staging (TNM), histological staging and malignancy scores.
References1. Pinêra K, Nogueira AC, Consolaro A. Determinação do teor alcoólico de anti-sépticos bucais e carcinogênese bucal química. Rev Bras Ciênc Estomatol 1996; 1: 13-17.
2. Zavras AI, Douglass CW, Joshipura K, Wu T, Laskaris G, Petridou E, Dokianakis G, Segas J, Lefantzis D, Nomikos P, Wang YF, Diehl SR. Smoking and alcohol in the etiology of oral cancer: gender-specific risk profiles in the south of Greece. Oral Oncology 2001; 37: 28-35.
3. Millon RR, Cassisi NJ, Mancuso AA. Oral cavity. In: Millon RR, Cassisi NJ, eds. Management of head and neck cancer. 2nd ed., Philadelphia; 1994, p.321-39.
4. Costa ALL, Pereira JC, Nunes AAF, Arruda MLS. Correlação entre a classificação TNM, gradação histológica e classificação anatômica em carcinoma epidermóide oral. Pesqui Odontol Bras 2002; 16: 216-20.
5. Baatenburg RJ, Hermans J, Molennar J, Briaire JJ, Le Cessie S. Prediction of survival in patients with head and neck cancer. Indian J Pathol Microbiol 2001; 44: 257-9.
6. O'Sullivan B, Shah J. New TNM staging criteria for head and neck tumours. Semin Surg Oncol 2003; 21: 3-7.
7. Macfarlane GJ, Boyle P, Scully C. Oral cancer in Scotland: changing incidence and mortality. Br Med J 1992; 305: 1121-3.
8. Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 1992; 166: 375-81.
9. Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral diseases 1998; 4: 70-77.
10. Dib LL, Sabba LMB, Marques LA, Araújo NS. Fatores prognósticos em carcinoma de borda de língua: análise clínica e histopatológica. Acta Oncológica Brasileira 1994; 14: 88-93.
11. Bryne M, Koppang HS, Lilleng R. New malignancy grading is a better prognostic indicator than "Broders" grading in oral squamous cell carcinomas. J Oral Pathol. Med 1989; 18: 432-37.
12. Hermanck P, Sobin HL, Fleming DI. What do we need beyond TNM? Cancer 1996; 77: 185-92.
13. Neville BW, Damn DD, Allen CM, Bouquot JE. Patologia Oral e Maxilofacial. 1ª ed., Rio de Janeiro: Guanabara Koogan; 1998. p. 1-705.
14. Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer supplement 1997; 80: 1529-34.
15. Lopes MA, Nikitakis NG, Reynolds MA, Ord RA, Sauk J. Biomarkers predictive of lymphonode metastases in oral squamous cell carcinoma. J Oral Maxill Surg 2002; 60: 142-7.
16. Colella G, Gabriele M, Lanza A, Tartaro GP, Giorgetti G. Clinical evaluation of patients with squamous carcinoma of the oral cavity. Minerva Stomatologia 1999; 48: 319-23.
17. Oliver AJ, Helfrick JF, Gard D, Primary oral squamous cell carcinoma: A review of 92 cases. J Oral Maxillofacial Surg 1996; 54: 949-55.
18. Varela-Centelles PI, Seoane J, Vasquez FE, De la Cruz A, Ansejo GJA. Survival to oral cancer. A study of clinical risk markers with independent prognostic value. Bull Group Int Rech Sch Stomatol Odontol 2002; 44: 46-51.
19. Bundgaard T, Bentzen SM, Wild TJ, Sorensen FB, Sogaard H, Nielsen JE. Histopathologic, stereologic, epidemiologic and clinical parameters in the prognostic evaluation of squamous cell carcinoma of the oral cavity. Head & Neck 1996; 3: 142-52.
20. Anneroth G, Hansen LS. A methodological study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Res 1984; 5: 448-68.
21. Bryne M, Nielsen K, Koppang HS, Dabelsteen E. Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med 1991; 20: 369-72.
22. Brown B, Barnes L, Mazariegos J, Taylor F, Johnson J, Wagner RL. Prognostic factors in mobile tongue and floor of mouth carcinoma. J oral Maxillofac. Surg 1998; 56: 832-6.
23. Odell EW, Path MRC, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, Path FRC, Morgan PR. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas, Cancer 1994; 74: 789-93.
24. Subdo J, Bankfalvi A, Byrne M, Marcelpoil R, Boysen M, Piffko J, Hemmer J, Kraft K, Reith A. Prognostic value of graph theory-Based tissue architecture analysis in carcinoma of the tongue. Laboratory investigation 2000; 80: 1881-9.
25. Broders AC. Squamous epithelioma of the lip: a study of live hundred and thirty-seven cases. J Am Med Assoc 1920; 74: 656-64.
26. Jakobsson PA, Anneroth CM, Killander D. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol 1973; 12: 1-8.
27. Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand. Dent. Res 1987; 95: 229-49.


