INTRODUCTIONEven though it has been described for a long time in the literature, the effect of glucose metabolic disorders (GMD) over the cochlear-vestibular system is still reason for discussion among inner ear investigators. One of the reasons for such disagreement lies in the impossibility of observing in vivo histopathological affections to the labyrinth, a situation that limits the investigation of the topic. The confirmation of higher prevalence of GMD in patients with cochlear-vestibular disorders when compared to the general population 1-4 is only indirect evidence of its importance in clinical manifestations presented by these patients.
It seems logical that the labyrinth system, which depends on continuous supply of energy 5, suffers influenced of the circulating levels of glucose and hormones, which depend on the generation of energy by ATP. Literature data estimate that the occurrence of GMD is between 42% and 80% in patients with tinnitus and dizziness 2, 6, 7, whereas 2.5 to 15% of the population presents asymptomatic hypoglycemia 4 or some affection of glucose tolerance curves 6. In Brazil, GMD have already been considered the most frequent cause of labyrinthic dysfunctions of metabolic etiology 7, and according to a recent survey in our population, they were documented by clinical history and fast glucose levels in 13% of the patients with vestibular symptoms 1.
The reluctance of some researchers in accepting that GMD may be a causal factor of balance disorders is lack of objective documentation of its direct implication in the genesis of vestibular problems. In a clinical review on the topic, Rybak8 reinforced the need for objective demonstration of the influence of GMD over the functioning of inner ear, even though it is difficult to deny the symptomatological improvement once the right diet is recommended.
Among GMD currently accepted as responsible for labyrinthic affections we can include glucose metabolic disorders such as diabetes, reactive hypoglycemia and hyperinsulinemia 9-11.
Upon reviewing the basic literature, we can see that secondary symptoms to hypoglycemia are divided into neuroglicopenic symptoms (drop in concentration of glucose in the central nervous system - CNS), which comprises mental confusion, fatigue, convulsions, loss of consciousness and behavioral changes, and autonomic symptoms, which include adrenergic manifestations such as palpitations, tremors and anxiety together with cholinergic manifestations, characterized by sudoresis, paresthesia and hunger sensation 12. Moreover, other symptoms as referred, such as "empty head", or absence of them in cases of chronic hypoglycemia 13. Classically, the diagnosis of hypoglycemia is made by the Whipple triad: symptoms compatible with hypoglycemia, plasma glucose low concentration, and relieve of symptoms after increase in plasma glucose concentration 12.
The first scientific document to relate glucose metabolic dysfunctions and inner ear affections was made by Jordão in 186414, who observed a correlation between sensorineural deafness and diabetes and defined a link between hearing loss and hyperglycemia. In 1960, glucose was finally recognized as one of the main elements in the maintenance of good functional inner ear activity 15. From then on, many authors documented vestibular cochlear affections secondary to diabetes mellitus and hyperinsulinemia 9,17-19.
Experimental EvidenceThe labyrinth is particularly sensitive to small variations of glucose and insulin plasma levels and the best evidence of this fact is the presence of insulin receptors in the endolymphatic sac 20 and glucose transporters in stria vascularis 21. Hypoglycemia, that is, the presence of high levels of insulin, interfere in the enzymatic activity responsible for the maintenance of endocochlear potential 9, 22. An objective demonstration of GMD in the vestibular organ of rats by means of evoked potentials was recently published by Perez et al .18.
In cases of diabetes mellitus, the abnormalities histologically observed are microangiopathy and peripheral neuropathy, responsible for difficulty in extremity blood flow and irregular supply of glucose 1. Some authors related minimum cell affections and functional commitment of central labyrinthic pathways as complications of initial diabetes mellitus, which do not present correlation with neuropathy or microangiopathy 10,17,19.
The importance of aerobic metabolism of glucose in maintenance of endolymphatic potential has already been experimentally documented 23. Even though hair cells may use other substrates to maintain the endolymphatic potential such as glutamate, piruvate and fumarate, none of them is as efficient as glucose 24, 25. It may also be detected by the presence of glycogen in stria vascularis but this alternative source of energy does not support the high demand required to maintain this potential in the absence of glucose 26.
The importance of the presence of glucose in maintaining energy levels in the inner ear may be observed by the facilitated form of access, through the scala vestibularis, probably through blood-perilymph barrier 27. The probable transporter is GLUT-1, present in large quantities in stria vascularis and dark cells, areas of high metabolic activity 21,28.
The true role that insulin performs in the operation of the inner ear is still obscure. Its interference in cochlear microphonics has already been known for decades 15 and according to some recent studies, insulin seems to be related to protein synthesis and not to glucose uptake 29.
Clinical DocumentationThe onset of Computed Dynamic Posturography (CDP) has complemented the classical battery of tests for otoneurological diagnosis and opens a new line of investigation of dizziness in patients that present complaints related to body balance not diagnosed by the conventional test battery. It is a computed system that allows isolation and quantification of the participation of vestibular, visual and somatosensorial information, as well as sensorial integration, in maintaining body balance. The basic test run by CDP, Sensory Organization Test (SOT) provides information about organization and coordination of the motor response evoked by stimuli received in supine position 3.
The device has a reference surface in which the patient stands up. This plan has pressure sensors that are activated depending on weight variations over the points of the foot in response to body displacement. The reference surface is surrounded by a mobile visual field, as if it were a telephone booth, which suffers anterior-posterior displacement, varying visual information.
Its use is particularly important as a follow-up test, which allows follow-up and assessment of the results of a specific treatment devised 30.
OBJECTIVEOur objective was to report the serial results of the Sensory Organization Test in the follow-up of patients with GMD treated with scheduled diet and glucose restriction.
MATERIAL AND METHODRetrospective descriptive study of 21 cases of patients with body balance disorders and GMD seen in the ambulatory of Otoneurology, Department of Otorhinolaryngology, FMUSP.
Based on clinical suspicion of GMD and normal fast glucose levels, patients were asked to undergo 3-hour glucose tolerance curve with insulinemia. For clinical diagnosis of GMD, we considered clear correlation between episodes of dizziness and food intake, headache, instability, persistent feeling of empty head, need to take sweet foods and family history of diabetes.
The normal range considered for the glucose-insulin curve were second-hour glucose above 145mg/dl; glucose level below 55mg/dl any time in the exam; fast insulin above 50U/ml, and sum of 2nd and 3rd insulinemia above 75U/ml3.
All patients were submitted to otoneurological assessment that included clinical history, ENT examination, cranial nerve test, cerebellar tests, electronystagmography (ENG), and computed dynamic posturography before and after treatment.
Based on diagnosis of GMD we recommended diet 11 during a period of 40 +/-10 days. Diet instructions are shown in Table 1.
During the study we did not use any type of drug that could affect the vestibular system.
We considered as studied variables the results obtained in the 6 conditions studied by the Sensory Organization Test of the Computed Dynamic Posturography, as well as the final equilibrium score (ES).
Statistical analysis of means obtained was made using t Student test. The level of significance was p<0.05, according to the standard adopted in biological studies.
RESULTSOur sample comprised 5 men and 16 women, mean age of 41.55 years and SD of 16.48 years.
Means before and after diet were 91.49+/- 1.65 and 93.84 +/- 1.68 for condition 1 (t=0.02, p>0.05); 86.43 +/- 0.94 and 90.61 +/- 5.46 for condition 2 (t=0.11, p>0.05); 86.47 +/- 8.7 and 90.92 +/- 6.18 for condition 3 (t=0.00, p>0.05); 69.61 +/- 16.29 and 79.96 +/- 8.62 for condition 4 (t=0.00, p>0.05); 48.81 +/- 16.35 and 66.79 +/- 11.13 for condition 5 (t= 4.63, p<0.05*); 47.71 +/- 19.01 and 60.48 +/- 14.82 for condition 6 (t= 0.004, p>0.05); 66.6 +/- 11.12 and 77.15 +/- 18.36 for equilibrium score (t= 9.37, p<0.05*). Variation was significant for condition 5 and equilibrium score (ES).
The variation of means obtained before and after the diet can be observed in Graph 1. The better the balance, the closer to 100%.
As to falls, we detected reduction of number of falls in 4 patients after the diet started, whereas two patients did not fall in the first exam, but fell in the second exam. There was no statistically significant difference between the groups (t= 0.05, p>0.05).
DISCUSSIONOur main purpose in this project was to present quantitative data concerning the effects of scheduled diet with glucose restriction to treat patients with GMD. This interest was triggered by previous studies in which we detected high prevalence of GMD in our ambulatory population who had cochlear-vestibular symptoms1,2.
Our routine includes fast glucose as screening test for all patients that present cochlear-vestibular complaints; however, if there is suggestion of GMD, we order 3-hour glucose-insulinemia curve. We have noticed that when the history is suggestive of the disorder, almost all cases present curve abnormalities and even in the absence of laboratory data, the clinical history prevails and the diet is started, producing satisfactory results in most cases. In fact, we observed clinical improvement in almost all patients in our study, plus qualitative gain confirmed by quantitative improvement documented by CDP.
Based on different experimental trials on the effects of glucose over the inner ear, we can not deny that it is a fundamental element in the generation of endocochlear potential 20-23. However, there were some questions about potentiality of GMD as etiological factor of symptomatology related to body balance. So far, acceptable clinical manifestations such as those suggestive of reactive hypoglycemia, for example, comprise neuroglicopenic and autonomic symptoms, such as mental confusion, fainting, palpitations, sudoresis, etc 12. Some authors from the clinical area, however, accept that hypoglycemia may lead to the well known sensation of empty head, which many people with GMD refer as dizziness, even though they may be completely asymptomatic 13.
According to Fukuda22, the consumption of sucrose (refined sugar) has increased significantly in recent decades and our bodies have not had enough time to adapt to the ingested high amounts. The result of this inappropriate intake is hyperinsulinemia with consequent reactive hypoglycemia and symptoms such as headache, sleepiness, dizziness, etc. There may be episodes of vertigo with tinnitus, classical symptoms of Ménière syndrome, resulting from water retention and increase in endolymphatic pressure. In such cases, there are the common complaints of fluctuation, imbalance and oscillopsy in the period in-between crises. GMD may occur at any age, but it seems to be more prevalent in middle-aged women as confirmed by our sample.
Used as a good follow-up tool, CDP has proved to be a useful instrument to document variations of body balance that result from treating the patients 30. The main conditions that assess vestibular function are those that allow oscillation of the platform of force, that is, conditions 4, 5 and 6. In these situations, the physiological oscillation of the gravity center of the body displaces the support surface with body balance and forces the patient to use vestibular information to correct the ankle angle to avoid falling. In this situation, the vestibular system is an afferent system whose precision of information has impact over body stabilization. It happens because vestibular information is more reliable in view of the environment that provides conflicting afferent information when compared to visual and proprioceptive information 31, 32. This characteristic of the exam can be observed in Figure 1, which presents results of CDP of one of the treated cases. Condition 5 is the situation that best assesses the vestibular system alone, considering that the patients are over a free platform and do not have visual information - similarly to standing up in a boat with closed eyes. It is the condition that presented statistically significant variation before and after treatment, which strongly suggests that the vestibular system is the most affected in GMD.
It can be observed in Graph 1 that situations that provide oscillation of platform present greater variation before and after the diet. This improvement can be observed in the significant improvement of the final equilibrium score (ES), which corresponds to the weighted measure of all assessed situations. These findings state the impact of treatment in global sensorial organization and the improvement in body stability previously affected by vestibular dysfunction. Based on these data, we can infer that the restoration of vestibular function is sufficient condition to make clinical symptomatology disappears.
As to falls, we observed reduction of number of falls after treatment, even though it was not significant. This impact over falls can be directly related with appropriateness of vestibular information, considering that when compared to visual and proprioceptive systems, the vestibular system presents smaller latencies in triggering action potentials. This physiological characteristic is indispensable condition in the process of sensorial integration that determines body stabilization in erect position in situations that require reflex posture corrections, in view of variations of support surface and/or environmental light. The non-significant reduction of falls may be related to the small sample and we believe that it would have been statistically different if the studied sample had been larger.
CONCLUSIONWe concluded that the Sensory Organization Test proved to be a useful tool in documenting improvement of body balance in patients with GMD submitted to scheduled diet with glucose restriction.
REFERENCES1. Bittar RSM, Bottino MA, Zerati FE, Moraes CLO, Cunha AU, Bento RF. Prevalência das alterações metabólicas em pacientes portadores de queixas vestibulares. Rev. Bras. de Otorrinolaringologia 2003; 1:64-69.
2. Sanchez TG, Bento RF, Miniti A, Câmara J. Zumbido: Características e Epidemiologia. Experiência do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. Rev. Bras. de Otorrinolaringologia 1997; 63(3): 229-35.
3. Mangabeira-Albernaz PL, Fukuda Y. Glucose, Insulin and Inner Ear Pathology. Acta Otolaryngol 1984 (Stockh) 97:496-501.
4. Charles DA, Barber HO, Hope-Gill HF. Blood glucose and insulin levels thyroid function and serology in Meniere's disease recurrent vestibulopathy and psychogenic vertigo. J Otolaryngol 1979; 8 (4):347-53.
5. Armour G, Mhaskar Y, Rybak L, Dunaway G. Alteration on 6-phosphofructo-1-kinase subunits during neonatal maturation of the rat cochlear cells. Hear Res 2001; 151(1-2):149-56.
6. Weille FL. Hypoglycemia in Meniere Disease. Arch Otolaryngol 1968; 87:129-31.
7. Albernaz PLM. Doenças metabólicas da orelha interna. RBM-Otorrinolaringologia 1995; 2 (1):18-22.
8. Rybak LP. Metabolic disorders of the vestibular system. Otolaryngol Head Neck Surg 1995; 112 (1):128-32.
9. Doroszewska G, Kazmierczak H. Hyperinsulinemia in vertigo tinnitus and hearing loss. Otolaryngol Pol 2002; 56(1):57-62.
10. Lisowska G, Namyslowski G, Morawski K Strojek K. Early identification of hearing impairment in patients with type 1 diabetes mellitus. Otol Neurotol 2001; 22(3):316-20.
11. Bittar RSM, Sanchez TG, Santoro PP, Medeiros IRT. O metabolismo da glicose e o ouvido interno. Arquivos da Fundação Otorrinolaringologia 1998; 2(1):4-8.
12. Foster DW. Hypoglycemia in Harrison's Principles of Internal Medicine. 14a. ed. McGraw-Hill; 1999. p. 2081-7.
13. Becker KL. Principles and Practice of Endocrinology and Metabolism. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2002.
14. Jordão apud Rust KR, Prazma J, Triana RJ, Michaelis OE, Pillsbury HC. Inner ear damage secondary to diabetes mellitus. Arch Oto Head Neck Surg 1992; 118:397-400.
15. Wang C, Crapo LM. The epidemiology of thyroid disease and implications for screening. Endocrinol Metab Clin North Am 1997; 26(1):189-218.
16. Koide Y, Tajima S, Yoshida M, Konno M. Biochemical changes in the inner ear induced by insulin in relation to the cochlear microphonics. Ann. Otol. Rhin. Laryngol 1960; 69:1083-97.
17. Orts Alborch M, Morant Ventura A, Garcia Callejo J, Perez Del Valle B, Lorente R, Marco Algarra J. The study of otoacoustic emissions in diabetes mellitus. Acta Otorrinolaringol Esp 1998; 49(1):25-8.
18. Hosch H, Ottaviani F. Otoacoustic emissions in diabetic patients with normal hearing. Schweiz Med Wochenschr 2000; 125:83S-85S.
19. Perez R, Ziv E, Freeman S, Sichel JY, Sohmer H. Vestibular end-organ impairment in animal model of type 2 diabetes mellitus. Laryngoscope 2001; 111(1):110-3.
20. Lisowska G, Namyslowski G, Morawski K Strojek K. Cochlear dysfunction and diabetic microangiopathy. Scand. Audiol. Suppl 2001; 52:199-203.
21. Knight LC, Saeed SR, Hradek GT, Schindler RA. Insulin receptors on the endolymphatic sac: an autoradiographic study. Laryngoscope 1995; 105(6):635-8.
22. Yoshihara T, Satoh M, Yamamura Y, Itoh H, Ishii T. Ultrastructural localization of glucose transporter 1 (GLUT1) in guinea pig stria vascularis and vestibular dark cell areas: an immunologic study. Acta Otolaryngol 1999; 119(3):336-40.
23. Fukuda Y. Açúcar amigo ou vilão? Barueri, SP: Manole; 2004.
24. Puschner B, Schacht J. Energy metabolism in cochlear outer cells in vitro. Hear Res 2001; 114(1-2):102-6.
25. Kambaiashi J, Kobaiashi T, Marcus NY, Demott JE, Thalmann R. Minimal concentrations of metabolic substrates capable of supporting cochlear potentials. Hear Res 1982; 7(1):105-14.
26. Marcus DC, Thalmann R, Marcus NY. Respiratory rate and ATP content of stria vascularis of guinea pig in vivo. Laryngoscope 1978; 88(11): 1825-35.
27. Kambaiashi J, Kobaiashi T, Demott JE, Marcus NY, Thalmann I Thalmann R. Effect of substrate free vascular perfusion upon cochlear potentials and glycogen of the stria vascularis. Hear Res 1982; 6(2):223-40.
28. Ferrary E, Sterkers O, Saumon G, Tran Ba Huy P, Amiel C. Facilitated transfer of glucose from blood into perilymph in the rat cochlea. Am J Physiol 1987; 253(1 Pt 2):59-65.
29. Nakazawa K, Spicer SS, Schulte BA. Postnatal expression of the facilitated glucose transporter GLUT 5 in gerbil outer hair cells. Hear Res 1995; 82(1):93-9.
30. Wang S, Schacht J. Insulin stimulates protein synthesis and phospholipid signaling systems but does not regulate glucose uptake in the inner ear. Hear Res 1990; 47(1-2):53-61.
31. Black FO. What can posturography tell us about vestibular function. Ann N Y Sci 940:446-64 2001.
32. Herdman SJ, Sandusky AL, Hain TC, Zee DS, Tusa RJ. Characteristics of postural stability in patients with aminoglycoside toxicity. Jl Vest Res 1994; 4:71-80.
33. Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol Head Neck Surg 1992; 106:175-180.
Table 1. Diet guidelines provided to patients diagnosed with glucose metabolic disorders.
- The diet should be divided into 3-hour intervals. Make smaller and more frequent meals.
- Regular white sugar should be replaced by sweetener.
- Replace regular rolls for diet, light or gluten bread.
- The effects of fruits such as grapes, figs and papaya should be carefully watched because they are rich in carbohydrates.
- Smoking and alcohol intake are forbidden.
- Caffeine should be restricted to 250 mg/day, that is, 3 small cups of coffee. As an alternative, decaffeinated coffee can be taken - 6 small cups a day.
- Soft drinks should be diet, but cola-like and guarana soft drinks are not allowed because of high caffeine content.
- Chocolate, black tea and mate should be avoided because they are CNS stimulants.
- Physical activity is highly recommended. In addition to relieving stress, it helps in lipid metabolism and circulation, positively interfering in vestibular rehabilitation. Walking is the best option.
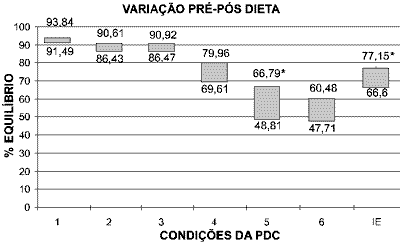 Graph 1.
Graph 1. Variation of final means for each condition of CDP obtained in patients with GMD before (inferior part) and after (upper part) of diet. Perfect balance is considered 100%, whereas zero represents decrease. Significant values are marked (*).
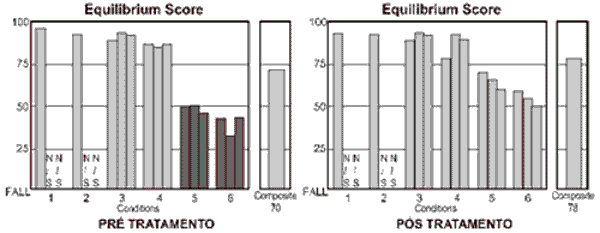 Figure 1.
Figure 1. Example of exam of patient with GMD before and after scheduled diet with glucose restriction. Dark bars correspond to responses below the normal range.


