INTRODUCTIONThe diagnosis of myoepithelioma is not frequent and it is found in approximately 1% of salivary gland tumors. The progression is benign and was described for the first time by Sheldon in 1943. In major salivary glands it prefers the parotid and in minor salivary glands, the palate region. It is believed that there is a spectrum of benign tumors comprising predominantly myoepithelial cells, but that presents different histological variations, differentiating them. On the one side we would have myoepithelioma, and on the other side, basal cell adenoma. Pleomorphic adenoma would be in the middle of the spectrum. Despite the fact that variations in architecture and cytology of these tumors are well defined, they lead to difficulties in terminology and diagnosis.
CASE REPORTCTC, 58-year-old female patient, came to the medical visit complaining of swallowing difficulties, no pain, followed by muffled voice. She also reported cough and choking sensation when lying on her back. The clinical picture had progressed for 3 years, and symptoms had worsened. She did not report alcohol abuse or smoking.
Oral cavity examination showed hardened tumor of approximately 3cm that was revealed to the oropharynx once the patient protruded her tongue. It had a rounded and well-delimited aspect. Superior surface presented slightly ulcerated and had white areas. It was not possible to define the exact point of implantation of the tumor under indirect laryngoscopy with mirror.
Using fibronasopharyngolaryngoscopy we noticed pediculated lesion, but the implantation basis was wide and located on the tongue base, more to the left (Figure 1). Epiglottis, vocal fold and the other laryngeal structures were intact. The lesion measured approximately 3cm in diameter.
There were no abnormal neck lymph nodes upon palpation and the remaining ENT examination was normal. Preoperative hematological tests and neck ultrasound to check minor salivary glands and thyroid were uneventful.
We scheduled removal of the lesion with wide elliptical incision and wedge-like incision at the tongue base under general anesthesia, which was conducted without complications. There was complete regression of symptoms after the surgical procedure. The 12-month follow-up showed that the tongue base was healed, with no signs of local recurrence (Figure 2).
Microscopy of the clinical pathology was: histological sections of non-encapsulated neoplasm located on tongue submucosa, comprising ovoid or polygonal epithelioid cells and clear cells, with regular chromatin nuclei and eosinophilic nucleoli. The lesion showed occasional microcystic spaces and accumulation of hyaline material between cell islands and strings. There were no ductal formations. Mitosis figures were rare. Free surgical resection margins. Conclusion: Pattern of neoplasm compatible with myoepithelioma (Figure 3). To confirm the diagnosis we ordered immunohistochemical study of the specimen, which showed marked and diffuse positive result to S-100 protein (Figure 4).
DISCUSSIONThe presence of a tongue base tumor can suggest the presence of ectopic thyroid gland, whose incidence ranges from 1:3000 to 1:10000. The incidence is 7 times higher in women and the diagnosis is given mainly between the ages of 60 and 70 years. To smaller tumors, which cause few symptoms, there is the possibility of suppression therapy with thyroid hormones. To larger tumors, which cause severe dysphagia or upper airway obstruction, the treatment of choice is excision of the lesion, and if necessary, postoperative hormone replacement.
In this case report, the diagnosis of myoepithelioma of minor salivary gland was made based on clinical pathology and immunohistochemical study of the tumor after complete exeresis. Myoepithelial cells were of ectodermic origin and they work as contraction cells. They can be found in normal secretion tissues, such as minor and major salivary glands, lachrymal and sweat glands, mucous producing glands of the trachea and esophagus, breast and prostate 1. These cells are found in the nasal membrane epithelium, and involve acinus and ducts of glands, assuming that they are responsible for expulsion and dissemination of secretion of acinus towards the duct.
It is a tumor that is not frequent in salivary glands. It has approximately 1% of incidence and it is mainly comprised of myoepithelial cells. Parotid gland and palate are the preferred sites and correspond to 2/3 of all the cases 2. The incidence of the disease is practically the same between the genders, or tends to be slightly higher among women. The age range is from 8 to 86 years, especially at the age of 50 years. It evolves clinically as a slow growth tumor and its size varies from 1 to 5cm in diameter. Normally it is finely encapsulated and well delimited. In tumors originated from the palate, the capsule may be absent, but it still remains well delimited 1. Its color is whitish (or pinkish, or grayish), non-painful, does not form ulcers (when located in the palate), and does not cause facial nerve paralysis (when located in the parotid) 2.
Cell architecture of myoepithelioma can have solid, mixoid or reticular pattern. Cell types found are spindle (more frequently in parotid tumors), plasmocytoid (more frequent in minor salivary glands), and less frequently, clear and epitheliod cells. Architecture and cell type do not change the prognosis of the disease 2-5.
The main immunohistochemical marker of myoepithelioma is S-100 protein and the diagnosis is hardly ever made when its result is negative 5. Leiomyoma and squamous cell neoplasm are negative for S-100 protein. Schwanomas are positive, but they present their own histological characteristics. Polymorphous adenocarcinomas can contain areas of myoepithelial differentiation that would be detected by hematoxylin-eosin staining and in immunohistochemical study, but differently from well delimited myoepitheliomas, they are diffusely invasive 3.
The presence of cell pleomorphism, increase in mitotic activity, necrosis foci, and tissue invasion are characteristics that suggest malignancy, such as for example malignant myoepithelioma of salivary glands (also known as myoepithelial carcinomas)6. It is a rare tumor and it presents intense mitotic activity (more than 7 mitosis figures/field, with 400x enlargement). It may originate from the malignant transformation of pleomorphic adenoma and myoepithelioma. Even though it is positive for S-100 protein, it is different from myoepithelioma because it is not limited and because of its high cell activity 4.
Even though pleomorphic adenoma is the main differential diagnosis with myoepithelioma both clinically and histologically, other tongue base tumors, of non-myoepithelial origin, should be excluded. The presence of a tumor in this location may suggest the presence of ectopic thyroid gland, whose incidence ranges from 1:3000 to 1:10000. The incidence is 7 times greater in women, and the diagnosis is given especially between the ages of 60 and 70 years. The assessment should be careful, given that 70% of these ectopic glands are producers of thyroid hormones. The type of treatment depends on symptom severity, size of ectopic gland, and whether it produces hormones or not, which causes few symptoms, because then there is the possibility of using suppression therapy with hormones. To larger tumors that cause severe dysphagia and upper airway obstruction, the treatment of choice is excision of lesion and if necessary postoperative hormone replacement therapy 1,8 .
Pleomorphic adenoma is the main differential diagnosis of myoepithelioma. To Simpson et al., 19959, it is in the middle of the benign salivary tumor spectrum, in which on the one side we have myoepithelioma and on the other, basal cell adenoma. Myoepithelioma is almost completely formed of myoepithelial cells and ductal formations are absent, or are very rare (present in less than 5% of the examined field). In pleomorphic adenoma, myoepithelial cells are present in varied number (occasionally, their disposition in pleomorphic adenoma can be similar to that of myoepithelioma) and ductal formations are innumerous 2,3,5,8. Both tumors present similar prognosis, which is good if treated through complete surgical excision.
CLOSING REMARKSAfter 12-month postoperative assessment, the patient was asymptomatic and videolaryngoscopy exam showed that the site where the tumor had been had no signs of recurrence. This progression is in agreement with the literature, which considers exeresis of the lesion with free margins the treatment of choice 1.
REFERENCES1. Andrea Gallo, Francesca Leonetti, Elisabeta Torri, Valentina Manciocco, Marilia Simonelli, Marco De Vincentiis. Ectopic lingual thyroid as unusual cause of severe dysphagia. Dysphagia 2001; 16:220-3.
2. Barnes L, Appel BN, Perez H, El-Attar AM. Myoepitheliomas of the head and neck: Case report and review. Journal of Surgical Oncology 1985; 28:21-8.
3. Michal M, Skálová A, Simpson RHW, Rychterová V, Leivo I. Clear cell malignant myoepithelioma of salivary glands. Histopathology 1996; 28:309-15.
4. Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, Kondo Y, Nagao K. Salivary Gland Malignant Myoepithelioma. Cancer 1998; 83(7): 1292-9.
5. Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: Report of 23 cases. Cancer 1982; 49: 562-72.
6. James T, Tighe JVP. A minor salivary gland tumour presenting with dysphagia. The Journal of Laryngology and Otology 1999; 113:569-72.
7. Rico NPM Rinkel, Johanes J Manni, Johan MH Van Der Beek. Ectopic thyroid tissue manifesting as a unique cause of an oropharyngeal mass. Otolaryngology- Head and Neck Surgery 2001; 124:340-1.
8. Kaneda T, Minami M, Ozawa K, Akimoto Y, Okada M, Yamamoto H, Suzuki H, Sasaki Y. Imaging tumors of the minor salivary glands. Oral Surgery Oral Medicine Oral Pathology 1994; 77:385-90.
9. Simpson RHW, Jones H, Beasley P. Benign myoepithelioma of the salivary glands: a true entity? Histopathology 1995; 27:1-9.
10. El-Naggar AK, Lovell M, Callender DL, Ordonez NG, Killary AM. Cytogenetic analysis of a primary salivary gland myoepithelioma. Cancer Genet Cytogenet 1999; 113:49-53.
11. Takai Y, Dardick I, Mackay A, Burford-Mason A, Mori M. Diagnostic criteria for neoplastic myoepithelial cells in pleomorphic adenomas and myoepitheliomas. Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endontic 1995; 79:330-41.
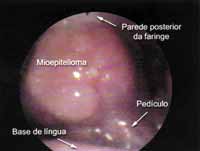 Figura 1
Figura 1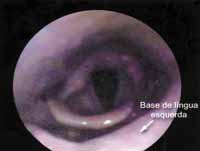 Figura 2
Figura 2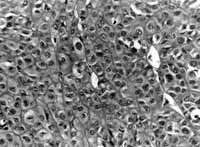 Figura 3
Figura 3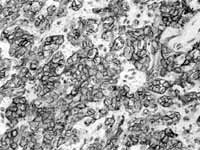 Figura 4
Figura 4 

