INTRODUCTIONHearing preservation is the current goal of modern surgery of acoustic neuroma (vestibular schwannoma). The evolution of surgical techniques enabled us to remove this tumor with minimal risk of neurological damage for the patients, preserving anatomic facial nerve integrity in most of the cases. Hearing criterion is useful and controversial; however, many authors consider that patients with SRT better than 50 dB and speech recognition above 50% are good candidates to surgical approaches that allow hearing preservation 1,2,3. There are two surgical approaches commonly used to remove such tumors with chances of hearing preservation: middle fossa approach and retrosigmoid approach. Both are widely used by enthusiastic advocates. 4
Retrosigmoid approach is limited by exposure of the internal acoustic canal (IAC) fundus and makes it difficult for the surgeon to perform total removal of the tumor, mainly if we take into account that facial nerve preservation in this approach has non-visible intrameatal portion before the removal of large parts of the tumor 5. The Middle Fossa Approach provides an excellent exposure of the IAC fundus and facial nerve, but it has anatomic constraints that prevent removal of tumors excessively extended to the posterior cerebral fossa. 6,7.
Pre-sigmoid retrolabyrinthine approach described by Hitselberger & Pulec in 19718 for functional surgery of the vestibular nerve has been used to remove vestibular schwannomas in cases selected by some surgeons 9-12. This has been our approach of choice for patients with useful preoperative hearing. The objective of the present study was to present 41 patients that had undergone vestibular exeresis through pre-sigmoid retrolabyrinthine approach (ARL), discussing the surgical technique adopted by us and the results of postoperative hearing preservation.
MATERIAL AND METHODForty-one patients with vestibular schwannoma and useful hearing have undergone surgery through pre-sigmoid retrolabyrinthine approach from January 1994 to February 2004 at Hospital das Clínicas -Medical School - USP.
Mean age was 37 years with 18 female and 23 male patients. Most of the tumors were referred to our service by ENT neurosurgeons from other Health Care Services in the country, with final diagnosis already defined.
The size of the tumor was measured using MRI axial cross section in T1 or axial CT scan of temporal bones. Then tumors were classified as intracanicular (IC) if they were exclusively confined to IAC. In case of tumor protruding to posterior brain fossa, the classification was based on the size of the larger diameter of the exclusive extra-meatal portion measured in centimeters, in compliance with the guidelines of the Committee on Hearing and Equilibrium of American Academy of Otolaryngology-Head and Neck Surgery (1995)14. Fifteen patients (36%) had only intracanicular tumors and 26 (64%) had tumors smaller than 1.5 cm.
According to the same Committee on Hearing and Equilibrium pre and postoperative hearing was classified in four classes (Table 1). All patients underwent pure tone and vocal audiometry at least one week before the surgery. In our group only 11 patients (26%) were classified as Class A, whereas most of them (74%) were classified as Class B.
Surgical Technique13
Patient was placed in dorsal horizontal supine position with lateral rotation of the head. Retro-auricular incision was performed in "C" shape at least 3 cm posteriorly to the projection of the sigmoid sinus. Mastoid and occipital regions should be extensively exposed. Mastoidectomy was performed with exposure of pre and retrosigmoid dura of the posterior brain fossa, all sigmoid sinuses (SS) until jugular bulb and medial dura of the middle fossa in all its extension to mastoid tegument (Figure 1). This extended dura exposure aimed at providing posterior retraction of the dura of the posterior fossa and of the sigmoid sinus increasing the area of visualization of the IAC. The posterior labyrinth, mainly the posterior and superior semicircular canals, should be skeletized until visualization of its membranous portion by transparency. It was critically important in one surgical approach in which the gain of few millimeters becomes indispensable. After such exposure, meatal plan dissection was initiated, always from the middle to the side. At this step it was not always possible to expose the side portion of the IAC dura and sectioning of the posterior fossa dura should be performed anteriorly to sigmoid sinus and expose posterior cerebral fossa (Figure 2). After emptying the spinal fluid from the cistern and making an extreme side rotation of the patient, it was possible to expose the remaining portion of the IAC. IAC was then opened in the longitudinal direction with exposure of its content, allowing tumor resection (Figure 3). With this approach we had partial control of the facial nerve, since it was not possible to dissect it before its labyrinth portion, such as the case in conventional translabyrinthine approach; however, since tumors removed through such approach are normally small they are not attached to the facial nerve, therefore could be anatomically preserved. Cochlear nerve should be positioned behind the tumor, from the surgeon's view; therefore, it should not pull the tumor during its removal before being completely loose (Figure 4).
This is a closed approach, similarly to conventional translabyrinthine approach. Temporal fascia was used to correct dura defect and abdominal fat was used to fill in the cavity, using biological glue.
This study was carried out prospectively and analyzed the following aspects in our management practice:
1. Likelihood of total removal of the tumor without requiring any modifications to surgical approach;
2. Intraoperative difficulties and complications;
3. Postoperative complications;
4. Hearing 90 days after surgery.
RESULTSTotal removal of the tumor was possible in all cases, as well as anatomic preservation of cochlear and facial nerves.
The intraoperative difficulty found involved the IAC fundus, which could not be approached in six patients, requiring a change to translabyrinthine approach for removal of side portion of the tumor. There were no intraoperative complications.
In early postoperative period (within the first week), three patients evolved to cerebrospinal fluid (CSF) leak, requiring new surgical approach to repair it. There were no significant complications such as intra-dura bleeding, meningitis or intracranial hypertension.
Audiologic evaluation performed 90 days after surgery reported 17 (41.6%) patients with anacusia, 14 (34.1%) maintained the same hearing class as preoperatively (Class A/B) and 10 (24.3%) patients kept some level of hearing (Class C/D) (Table 2).
DISCUSSIONThe possibility of hearing preservation in vestibular schwannoma (acoustic neuroma) surgery provides new treatment perspectives for these tumors. Major development in techniques and diagnoses, especially with popularization of MRI, has modified the natural course of vestibular schwannoma.
It is increasingly more common to have diagnosis of small tumors, with considerable impact in surgical outcome. Only 41 out of 273 cases of acoustic neuroma (vestibular schwannomas) that have undergone surgery in our health care service from 1994 to 2004 were operated through pre-sigmoid retrolabyrinthine approach (ARL), as most of the patients presented tumors larger than 1.5 and poor residual hearing.
Pre-sigmoid retrolabyrinthine approach was appropriate to view all IAC in 35 (85.5%) patients with subsequent total removal of the tumor. In the six cases in which it was not possible, the transformation into conventional translabyrinthine approach 15,16 solved the space issues of the fundus of the canal. As an approach that spares functional structures, pre-sigmoid retrolabyrinthine approach has anatomic limitations. In some cases it is not possible to approach IAC fundus even after retraction of the posterior dura and extreme side rotation of the patient, such as occurred in six of our cases. Elevated jugular bulb or limited space between sigmoid sinus and posterior semicircular canal were reasons for such anatomic limitation. Unfortunately, this anatomic configuration could not be foreseen before the surgery, not even in high quality imaging studies. The possibility of transforming the retrolabyrinthine approach into conventional translabyrinthine in case additional space is required should be explained to patients before surgery, since such possibility is a great advantage against those approaches that preserve hearing - such as middle fossa approach and retrosigmoid approach, because this change of approach is technically simple and natural.
The middle fossa approach has been used in several health centers as the first choice if the objective is hearing preservation. It also has its anatomic limitations such as impossibility of accessing the tumor projected to the posterior cerebral fossa. This technique has been recently extended and allowed the removal of larger tumors; however, posterior limitation still exists. In our opinion, it is an excellent option for skilled surgeons6,7,17.
The third alternative for hearing preservation in acoustic neuroma surgeries is the retrosigmoid approach. It offers an extended operative field, appropriate to remove tumors of any size18. The fact that it is basically a neurosurgical approach and requires long staying at the hospital made this approach be restricted to extremely large tumors (above 3.5 cm) in our service, situations in which surgery is performed together with a neurosurgeon. Currently, even in large tumors we use the extended translabyrinthine approach. The retrosigmoid approach is also anatomically limited to the IAC fundus by the posterior semicircular canal; it makes hearing preservation difficult in intra-canalicular tumors through this approach.
In our group of patients we removed the tumors completely without any major postoperative complication. The three cases of CSF leak were treated and this event was not more frequent than for other surgical approaches. The fact that there were no major complications can be only explained by the small size of tumors in this series of patients, however, it also shows perfect command of the surgical technique used.
Hearing preservation was possible in 34.1% of the cases to the same level as before the surgery (Class A/B). In 24.3 % of the patients, some hearing was preserved. This hearing preservation shows how important it is to know that the cochlear and facial nerves are anatomically preserved and can be used in the future for cochlear implant if the patient has hearing loss on the other ear. Nevertheless, it has a disadvantage of still being uncomfortable for some patients, particularly regarding tinnitus prevalence.
CONCLUSIONHearing preservation in selected cases of acoustic neuroma (vestibular schwannoma) is possible and should be one of the goals of this surgery. The retrolabyrinthine approach is an excellent option for those surgeons with practice in using conventional translabyrinthine approach, allowing safe removal of the tumor and anatomic preservation of cochlea and cochlear nerve.
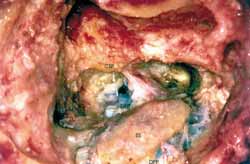 Figure 1.
Figure 1. Extended mastoid exposure allowing retraction of posterior dura.
CSP- Posterior semicircular canal
SS- Sigmoid sinus
DFP- Dura of posterior cerebral fossa
DFM- Dura of middle cerebral fossa
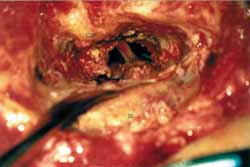 Figure 2.
Figure 2. Retraction of posterior dura and opening of the posterior cerebral dura providing the view to brain stem and 8th nerve.
CSP- Posterior Semicircular Canal
DFP- Dura of posterior cerebral fossa
FN- Nervous bundle
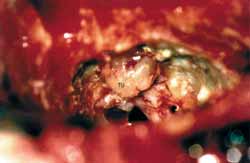 Figure 3.
Figure 3. Full exposure of IAC with tumor inside it.
CSP- posterior semicircular canal
TU- Tumor
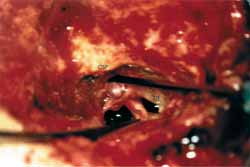 Figure 4.
Figure 4. Intact cochlear nerve after tumor resection.
CSP- posterior semicircular canal
NC- Cochlear Nerve
NF- Facial Nerve
align="center">
Table 1. Hearing Classification System recommended by the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology -Head and Neck Surgery.
 Table 2.
Table 2. Pre and postoperative tumor size and hearing.
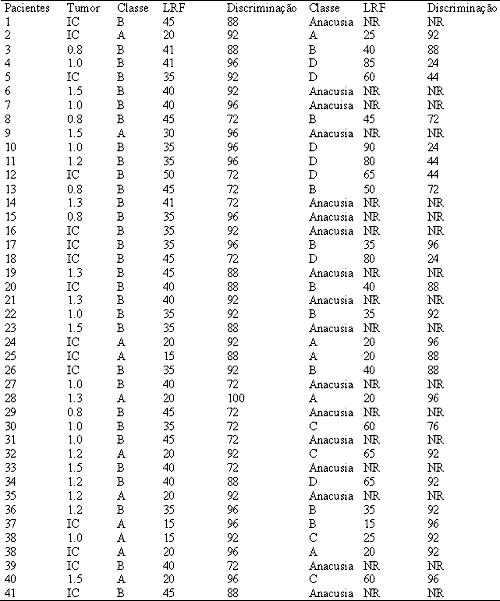
IC, Intracanalicular; LRF, Speech Discrimination Threshold; NR, None of the answers
1. Kanzaki J, Ogawa K, Inoue Y, et al. Quality of hearing preservation in acoustic neuroma surgery. Am J Otol 1988; 19: 644-8.
2. Molony T, Kwartler J A, House W F, Hitselberger WE. Extended Middle fossa and retrolabyrinthine approaches in acoustic neuroma surgery: case reports. Am J Otol 1992; 13; 360-3.
3. Ogawa K, Kanzaki J, O-Uchi T, et al. Preoperative findings and hearing preservation in acoustic neuroma surgery. Acta Otolaryngol 1991 (Stockh) suppl 487:30-5.
4. Jackler RK, Pitts L. Selection of surgical approaches to acoustic neuroma. Otolaryngologic Clinics of North America 1992; 25(I): 361-87.
5. Shelton C, Alavi S, Li J, Hitselberger WE. Modified retrosigmoid approach: use for selected acoustic tumor removal. Am J Otol 1995; 16: 664-8.
6. Brackmann DE, House III JR, Hitselberger WE. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuroma. Am J Otol 1994; 15: 614-9.
7. Bento RF, Brito Neto RV, Sanchez TG. A rapid and safe middle fossa approach to the geniculate ganglion and labyrinthine segment of the facial nerve. Ear Nose Throat 2002; 81: 320- 6.
8. Hitselberger WE, Pulec JL. Trigeminal nerve (posterior root) retrolabyrinthine selective section-operative procedure for intractable pain. Arch. Otolaryng 1972; 96:412-5.
9. Prades JM, Chelikh L, Merzougui N, et al. Voie d"abord rétro-labyrinthique öptimisée" Ann Otol Rhinol laryngol 1995; 112: 46-51.
10. Darrouzet V, Guerin J, Naaman A, et al. The widened retrolabyrinthine approach: a new concept in acoustic neuroma surgery. J neurosurg 1997; 86:812-21.
11. Brackmann, DE, Hitselberger WE. Retrolabyrinthine approach: technique and newer indications. Laryngoscope 1978; 88: 286-97
12. Darrouzet V, Guerin J, Portmann D. La voie rétrolabyrinthine élargie: application à la chirurgie du neurinome de l"acoustique. Rev Laryngol Otol Rhinol 1993; 114: 207-11.
13. Bento, RF, Brito Neto, RV, Miniti, A, Sanchez, TG The transmastoid retrolabyrinthine approach in vestibular schwannoma surgery. Otolaryngol Head Neck Surg 2002; 127: 437-41.
14. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg 1995; 95:179-80.
15. Bento RF, Miniti A, Bogar P- Experiência em 115 casos de cirurgia para exérese de neurinoma do acústico. Revista Brasileira de Otorrinolaringologia 1998; 64: 20-4.
16. Bento RF, Caropreso CA, Miniti A. A via translabiríntica na cirurgia do neuroma do acústico. Revista Brasileira de Otorrinolaringologia 1989; 55: 57-63.
17. Kanzaki J, Ogawa K, Inoue Y. Quality of hearing preservation in acoustic neuroma surgery. Am J Otol 1988; 19: 644-8.
18. Cohen NL, Hammerschlag P, Berg H. Acoustic neuroma surgery: an eclectic approach with emphasis on preservation of hearing. Ann Otol Rhinol laryngol 1986; 95: 21-7.


