INTRODUCTIONGiant cell reparative granuloma (GCRG), also known as giant cell central granuloma, considered an intra-bone tumor by the World Health Organization, is an uncommon, non-neoplastic tumor responsible for at least 7% of all expansive damage to the mandible, the most common location of the tumor 1. Less frequently, it may be found in the maxillary antrum and it is rarely presented with ethmoid involvement 8.
GCRG is an important differential diagnosis in mandible and sinusal expansive lesions, especially in children and adolescents.
GCRG should be differentiated from giant cell tumor, also named osteoclastoma, from aneurysmatic bone cyst and hyperparathyroid brown tumor. In fact, histopathological differentiation between GCRG and giant cell tumor may be difficult and in such situations, clinical data such as patients' age, clinical behavior of the tumor and response to treatment are important for diagnostic elucidation 22.
Until 1953, mandible expansive lesions with multinucleated giant cells (MNGC) were generically designated as giant cell tumors. In that year, Jaffè14 proposed the term giant cell reparative tumor of the mandible, recognizing it as a non-neoplastic bone disease, of benign curse, and it was believed that it resulted primarily from a process of tissue repair to a previous lesion, of alleged traumatic nature. Giant cell tumor, in turn, would be a true neoplasm, and even though it is histologically benign in most cases, it can suffer metastases especially to the lungs. Currently, it is known that both tumors may be locally aggressive 22.
Later, GCRG was described in many different articles in the paranasal sinuses 9, 18, temporal bone, orbit 12 and limb tubular bones 16. The differentiation between GCRG and giant cell tumor (GCT) has become well defined and widely accepted.
Even though they present histopathological similarities, GCRG and GCT normally manifest clinical and histological characteristic findings that support the differentiation between them, but they may vary significantly one from the other.
The purpose of the present study was to describe two cases of GCRG in male patients with maxilla-ethmoid and maxilla involvement, respectively, and to review the inherent characteristics of GCRG emphasizing differential diagnosis, especially with giant cell tumors.
LITERATURE REVIEWFew bone tumors have raised so much controversy in terminology as GCRG. Concerning the designation giant cell central granuloma, it is noticed that the term central promotes an important differentiation from other giant cell tumors (osteoclastoma), which normally affect long bones. However, GCRG has also been described in tubular bones of the hands and feet 16.
Conversely, the term reparative granuloma proposed by Jaffè based on the pathophysiogenic hypothesis of a post-trauma repair is still under discussion, However, it is not a true granuloma, because there is no true epithelioid cell, given that some authors believe that MNGC in fact present osteoclasts 13. However, the designation given by Jaffè is attractive and highly used, given that there is histological evidence suggestive of tissue repair, in which post-trauma intra-bone hemorrhage would lead to localized inflammation and subsequent development of tumor in predisposed subjects. According to this hypothesis, the hemosiderin found in macrophages, frequently found in these lesions, would be the result of intra-bone hemorrhage. Finally, the common finding of bone neoformation would be strong evidence of the tissue repair process. It is in fact not unexpected to find evident past history of local trauma or chronic sinusopathy.
In cases of paranasal sinuses GCRG, it is believed that a chronic inflammatory process rather than a non-traumatic process, is the most frequent factor associated with intra-bone hemorrhage 11 and subsequent reaction of MNGC.
Some typical histological findings are fibrous cell tissue associated with hemorrhagic foci and multinucleated giant cells 11. Occasional deposits of hemosiderin are common. The stroma of recent connective tissue has numerous elongated and spindle fibroblasts and some cystic formations. MNGC normally shows fewer nuclei compared to giant cell tumors.
Giant cell tumor, in turn, shows cytoplasmatic preponderance over cellularity and fibroblasts present marked cell predominance, in addition to rounded format. In CGT, the tissue is densely occupied by multinucleated giant cells surrounded by scarce spindle fibroblast stroma. Mitosis figures can also be observed, in contrast with GCRG, in which they are absent or rarely detected.
Conversely, aneurysmatic bone cyst (ABC) is considered a reparative bone tissue and it presents many characteristics in common with GCRG. However, it is more frequent in long bones and vertebrae. It is rarely developed in the facial skeleton, but it also has the mandible as preferred site of origin in the head. It is equally more frequent in the first and second decades of life and in female subjects. According to our best understanding, only two cases with ethmoidal involvement were described in the literature 3. Histologically, it is not a true cyst because it does not present epithelial recover 15. It is characterized by fibrous stroma that houses MNGC and multiple sinusoid and cavernous vascular spaces, responsible for abundant intraoperative bleeding. Some authors believe that this tumor may originate from other primary lesions such as GCRG, which owing to unknown reasons, suffers hemodynamic abnormalities that could lead to the vascular affections previously described 15.
Bone disease secondary to hyperparathyroidism of any etiology is normally associated with increase in bone reabsorption and osteopenia. However, in later stages, osteoclastic activity may result in development of multiple intra-bone cystic formations associated with hemorrhage foci and hemosiderin deposits. These lesions are known as hyperparathyroid brown tumor or cystic fibrous osteitis and should be included in the differential diagnosis of GCRG. Similarly to the previously mentioned tumors, it is more common in the mandible when affecting the head region. Serum levels of parathyroid hormone (PTH) and calcium are high in contrast with hypophosphatemia, resulting from a continuous process of bone demineralization.
CASE REPORTCase 1
Male 11-year-old patient, African-descendent, with history of proptosis and nasal obstruction on the right, progressive complaint for approximately 1 year. The patient did not report eye pain or diplopia. No past history of cranial-facial trauma or sinonasal inflammatory disease.
Upon physical examination, we detected gross facial deformity with marked proptosis on the right ocular globe and presence of tension upon palpation in the region of orbit supra-medial soft issue. Presence of ipsilateral conjuctival hyperemia. Bilaterally preserved extrinsic ocular movement. Anterior rhinoscopy revealed right nasal fossa obstruction caused by tumor. Remaining physical examination, including head and neck, did not show other abnormalities.
Paranasal sinuses Computed Tomography (CT scan) showed expansive lesion of regular contours and partially defined limits, measuring about 6.0cm in its longest diameter, comprising predominantly the right maxillary and ethmoid sinuses, displacing the ocular globe and the ipsilateral medial rectus muscle towards anterior and lateral direction. We also evidenced continuity solution of the tumor with the floor of the right frontal sinus and anterior wall of homolateral sphenoid sinus, with small extension to its interior. Right nasal fossa was partially filled by the tumor. Absence of contralateral involvement (Figures 1 and 2). CT scan characteristics suggested the ethmoid sinus as the origin site of the tumor.
Case 2
Male 9-year-old patient, African-descendent, with complaint of tumor in the left malar region for 2 years with progressive growth, non-painful, associated with facial deformity. The patient did not report nasal obstruction, rhinorrhea or epistaxis. He reported facial trauma approximately 3.5 years before on the left malar region.
Upon physical examination, we detected macromalar on the left. Absence of phlogistic signals. It was characterized as a hardened tumor upon palpation, non-painful, limited to the left maxillary region. Dental arch compatible with the age range. Hard palate had moderate bulging on the left. Anterior rhinoscopy showed progressive anterior-posterior narrowing of left nasal fossa, with medialization of lateral nasal wall, suggesting a consequence of the expansive ipsilateral maxillary process, in addition to mucus stasis.
Paranasal sinuses CT scan showed solid expansive formation of regular contours and well-defined limits, measuring approximately 3.6cm in its longest diameter, affecting the left maxillary sinus and homolateral alveolar process, with thinning of bone cortical without showing evidence of rupture, and heterogeneous density with areas suggestive of calcification and cystic formations. In the contralateral maxillary antrum floor we could see an image suggestive of mucous cyst (Figure 3).
In the two studied cases, routine lab tests and serum calcium were within the normal range.
We conducted surgical exeresis of tumors, performed without any events. However, it was necessary to remove the unstable dental units numbers 24, 26 and 27 (inserted) in the patient with maxillary tumor approached via Caldwell-Luc.
In the patient with unilateral pansinusal disease, external approach combining expansive Lynch incision and Caldwell-Luc approach was enough to provide satisfactory exposure of the tumor. The medial wall of the orbit was reconstructed with titanium prosthesis (Figure 4). On the second postoperative day, the patient presented with diplopia when looking to the left and diverging strabismus, which disappeared after the 24th postoperative day.
Clinical, endoscopic and CT scan follow-up of both patients was conducted for 2 years and did not show any events (Figure 5). Clinical pathology results showed that they were giant cell central granulomas, and these conclusions were confirmed by two other reference centers in clinical pathology in Brazil (Figures 6, 7 and 8).
DISCUSSIONIn the mandible, GCRG is twice more frequent in female subjects 21. It is more common in children, adolescents or young adults. Conversely, a GCT in patients aged below 18 years is exceptional and so far it has not been described in subjects aged below 10 years 11. Giant cell tumors (osteoclastoma) are more frequent in the third and fourth decades of life. In both studied cases, the age range of patients was in accordance with the classical descriptions.
GCRG is more frequent in the mandibular bone (2/3 of the cases) than in the maxilla and its is rarely found outside the cranial-facial skeleton, and only few cases have been reported in the frontal, ethmoid and temporal bones. GCT, conversely, typically affects the epiphysis of long bones. In the head, however, it is also more common in the mandible 5.
Both studied cases represented atypical locations of GCRG. According to the literature, to present, only 17 cases of ethmoid GCRG have been described 1,13,22, and only one of them was reported as a tumor limited to the ethmoid 1. On the other hand, maxillary involvement of the tumor has been well described in many different reports.
As to possible etiopathogenic factors previously described, only patients with GCRG circumscribed to maxillary sinus presents history of facial trauma.
The clinical picture may progress within few weeks or even years, normally associated with tumor expansion and their respective compressive effects over adjacent structures, with possibility of local discomfort, but pain is infrequent. Therefore, symptoms range depending on tumor location and aggressiveness. From the maxillary sinus, there is frequent involvement of pre-molar, molars and canines. Diplopia, proptosis, amaurosis, headache, nasal obstruction, epistaxis, ophthalmoplegia, cranial nerve affections and facial deformities are some of the possible and nonspecific symptoms related with cranial facial tumors.
Owing to the exuberance of the clinical picture, both cases suggested the presence of extensive tumors, which was confirmed by radiological and intraoperative findings.
Imaging exams, especially CT scan, are very useful in defining lesion location and extension. In the studied cases, the finding of expansive lesion with heterogeneous image and soft part density in the CT scan, with well-defined limits and calcification areas, possibly caused by bone neoformation, were in agreement with previous studies. Magnetic resonance image is particularly valuable to investigate intracranial tumor invasion.
Clinical pathology has concluded the final diagnosis of both cases. Whenever possible, the tumor should be submitted to preoperative biopsy and the specimen sent to clinical pathology analysis. Recently, some authors have described immunocytochemical analysis with tumor needle aspiration to diagnosis primary or recurrent disease 6.
Most authors agree that the treatment of choice for GCRG is surgery, except for cases considered non-operable or recurrent, which are not frequent situations 23. Postoperative recurrence ranges from 4 to 12%, and it is normally attributed to incomplete tumor resection 1. On the other hand, GCT presents high rate of recurrence after surgery or radiotherapy and it can originate metastases. It is known that GCT does not regress spontaneously without treatment, whereas spontaneous improvement of GCRG has already been described 22.
Surgical treatment may be conducted by curettage or en bloc resection, but with possibility of recurrence, regardless of the technique used. Recent studies associated cortical bone perforation of the mandible for histopathological analysis with higher risk of recurrence of GCRG in this site 17.
Anti-angiogenic therapy with interferon alpha 2a, well established in the treatment of hemangiomas, has presented promising results as treatment alternative to GCRG in non-operable or recurrent cases 7. In case of such limitations, some authors have advocated radiotherapy use, with variable results. However, sarcomatous malignant transformation of GCRG has been reported in patients submitted to radiotherapy 2.
As seen in most cases with GCRG, the studied patients did not require postoperative intervention, either surgical or clinical, and to the present moment, no recurrence has been observed.
CLOSING REMARKSEven though rare, the possibility of a GCRG affect the paranasal sinuses should be considered in the differential diagnosis of tumors in this location; clinical and imaging data are nonspecific and diagnostic confirmation should be made by clinical pathology. The treatment of choice is surgical removal, given that radiotherapy or anti-angiogenic therapy are reserved to non-operable or recurrent cases. Since it is a benign lesion, correct early diagnosis prevents mutilating radical treatment approaches.
REFERENCES1. Arda, HN; Karakus, MF; Ozcan, M; Arda, N; Gun, T. Giant cell reparative granuloma originating from the ethmoid sinus. Int J Pediatr Otorhinolaryngol 2003;67(1):83-7.
2. Austin, LT; Dahlin, DC; Royer, RQ. Giant cell granuloma and related conditions affecting jaw bones: an analysis of 38 cases. Oral Surg Oral Med Oral Pathol 1959; 12: 1285-90.
3. Baker, HL; Papsidero, MJ; Batsakis, JG; Krause, CJ. Aneurysmal bone cyst of the ethmoid. Head Neck Surg 1982; 5: 177-80.
4. Bodner, L; Bar-Ziv, J. Radiographic features of central giant cell granuloma of the jaws in children. Pediatr Radiol 1996; 26:148-151.
5. Burman, JA; Benson, J; Cohen, I. Giant cell tumor of the ethmoid sinuses: diagnostic dilemma. Laryngoscope 1979; 89: 1415-24.
6. Castro, WH; Filho, ECS; Souza, PEA; Gomez, RS. Imunocytochemistry of fine-needle aspirates from central giant cell granuloma. Br J Oral Maxillo Facial Sur 1998; 36: 301-03.
7. Collins, A. Experience with anti-angiogenic therapy of giant cell granuloma of the facial bones. Ann R Aust Coll Dent Surg 2000; 15: 170-5.
8. Felsberg, GJ; Tien, RD; McLendon, LE. Frontoethmoidal giant cell reparative granuloma. AJNR Am J Neuroradiol 1995; 16(7): 1551-4.
9. Friedberg, SA; Eisenstein, Re; Linden, JW. Giant cell lesions involving the nasal accessory sinus. Laryngoscope 1969; 79: 763-76.
10. Hamlin, WB; Lund, PK. "Giant cell tumors" of the mandible and facial bones. Arch Otolaryngol 1967; 86: 658-65.
11. Hirsh, S; Katz, A. Giant cell reparative granuloma outside the jaw-bone. Hum Pathol 1974; 5: 171-181.
12. Hoopes, PC; Anderson, RL; Blodi, FC. Giant cell(reparative) granuloma of the orbit. Ophthalmology 1981; 88: 1361-6.
13. Hyver, SW; Ellis, DS; Stewart, WB; Spencer, WH; Bartlett, PC. Sino-orbital giant cell reparative granuloma. Ophthal Plast Reconstr Surg 1998;14(3):178-81.
14. Jaffè, HL. Giant cell reparative granuloma, traumatic bone cyst, end fibrous(fibro-osseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 1953; 6: 159-75.
15. Larsen, PE; Hegtvedt, AK. Odontogenesis and odontogenic cysts and tumors. In: Cummings, CW. Otolaryngology-Head and Neck Surgery 1998, terceira edição, Mosby, pp. 1572-73.
16. Lorenzo, JC; Korfman, HD. Giant cell reparative granuloma of the short tubular bones of the hands and feet. Am J Surg 1980; 4: 551-63.
17. Minic, A; Stajcic, Z. Prognostic significance of cortical perforation in recurrence of central cell granulomas of the jaws. J Cranio Maxillo Facial Sur 1996; 24: 104-08.
18. Schlorf, RA; Koop, SH. Maxillary giant cell reparative granuloma. Laryngoscope 1977; 87: 10-7.
19. Upchurch, KS; Lee, SS; Schiller, AL; Rosenthal, DI; Canpion, EW; Crane, SM. Giant cell reparative of Paget´s disease of bone. Ann Intern Med 1983; 98: 35-40.
20. Kaffe, I e col. Radiographic features of central giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 1996; 81: 720-6.
21. Waldron, CA; Shafer, WG. The central giant cell reparative granuloma of the jaws: an analysis of the 38 cases. Am J Clin Pathol 1966, 45: 437-47.
22. Wiatrak, BJ; Gluckman, JL; Fabian, RL. Giant cell reparative granuloma of the ethmoid sinus. Otolaryngol Head Neck Surg 1987; 97: 504-509.
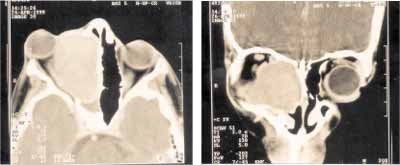 Figures 1 and 2-
Figures 1 and 2- Axial and coronal sections Computed Tomography revealed expansive formation with soft part intermediate density involving the right ethmoid and maxillary sinuses beyond the extension to the homolateral nasal fossa. It showed marked proptosis, papyraceous lamina erosion and lateral and anterior displacement of the ocular globe and medial rectus muscle on the right, in addition to obstructive sphenoid sinusopathy.
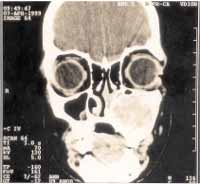 Figure 3-
Figure 3- CT scan images evidenced expansible lesion on the left maxillary sinus with heterogeneous density and calcification areas. We observed significant extension to the adjacent alveolar process.
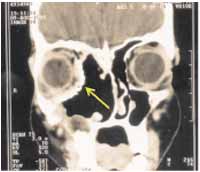 Figure 4-
Figure 4-Paranasal sinuses CT scan on the 14th postoperative day, highlighting the titanium prosthesis that replaced the medial bone wall of the orbit (yellow arrow).
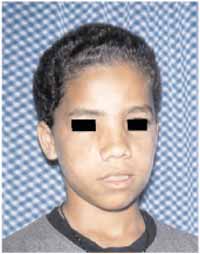 Figure 5-
Figure 5- facial aspect on the second postoperative month.
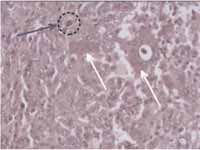 Figure 6-
Figure 6- histopathology showed tissue with spindle, elongated and abundant fibroblast stroma that surrounded the multinucleated giant cells (white arrow). We observed hemosiderin aggregates inside the macrophages (circle marked with the arrow) (HE, 400x).
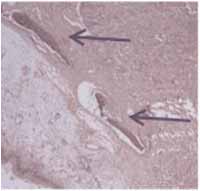 Figure 7-
Figure 7- blue arrows indicate neoformed bone tissue (HE, 100x).
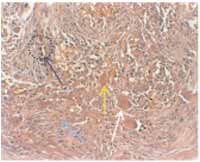 Figure 8-
Figure 8- histopathology showed abundant stroma rich in spindle fibroblasts (blue arrow), multinucleated giant cells (white arrow), macrophages containing hemosiderin (dotted circle, with arrow) and hemorrhagic aggregates (yellow arrow) (HE, 200x).
(1) Master studies under course, Center of Post-Graduation in Medicine and Health, Federal University of Bahia. Former resident physician, Service of Otorhinolaryngology, Hospital das Clínicas, Federal University of Bahia.
(2) Former intern physician, Service of Otorhinolaryngology, Hospital das Clínicas, Federal University of Bahia.
(3) Coordinator of the Division of Head and Neck Surgery, Service of Otorhinolaryngology, Hospital das Clínicas, Federal University of Bahia.
(4) Ph.D., Professor, Discipline of Otorhinolaryngology, Medical School, Federal University of Bahia, and Head of the Service of Otorhinolaryngology, Hospital das Clínicas, Federal University of Bahia.
Study conducted at the Service of Otorhinolaryngology, Hospital das Clínicas, Federal University of Bahia.
Address correspondence to: Fernando P. G. Sobrinho, Cd. Rec. dos Pássaros, R3, B29-A, Ap.301, Cabula, CEP: 41150-050 - Salvador-Ba. Tel (55 71) 257-0226.
E-mail: fpgsobrinho@bol.com.br


