INTRODUCTIONSinonasal polyposis (SNP) is a term used to describe an inflammatory condition of the mucosa surface of the nasal mucosa and paranasal sinuses 1, 2. Its etiology has been widely discussed, and there are many theories about its origin 1-7, which has been associated with many different diseases, such as cystic fibrosis 4, 8, aspirin triad 4, 6, 9, mucoviscidosis 2, 4, 10 and fungal sinusitis 11, 12.
The main symptom of this disease consists of progressive nasal obstruction (100%), which takes the patient to looking for the ENT physician. They also manifest rhinorrhea (69%), olfaction and taste abnormalities 1, and repetitive rhinosinusitis 13 (present in 100% of these patients).
Polyps originate from the middle concha, middle meatus and ethmoid sinus 4, 14. They affect primarily the ethmoid cells in diffuse and bilateral impairment, causing secondary mucous thickness and/or retention of secretion in other cavities.
The physical examination of nasal fossa shows multiple, bilateral, pale polypoid masses that can be restricted to the middle and upper portion of the nasal fossa or occupy them completely. It is important to bear in mind that other nasal fossa and paranasal sinuses tumors can be macroscopically similar to SNP, even though they are rarely bilateral.
Sinonasal surgery to remove polyps is indicated to immediately relieve the patients in advanced stages or when clinical treatment is not effective. In pre-surgical assessment, computed tomography scan (CT) becomes indispensable 15, since it is ideal to delimit the delicate infundibulum-bone complex, bone structure of orbital lamina, orbital floor, cribiform lamina and the anatomical variations 16.
The retained discharge in the paranasal sinuses secondary to rhinosinusitis that follows the disease hinders SNP staging. Density of the discharge is similar to polyps, which absorbs much liquid owing to its specific polysaccharide material 1. In addition to this fact, polyps may not be identified in the beginning of the disease or be obscure if intranasal steroids have been used 10, 17. Nasal endoscopy, despite being an important diagnosis for small meatal polyps, does not provide information about presence and extension of the disease to the ethmoid sinuses or to other sinuses. This is the reason why it is important to use CT scan to stage and support the diagnosis of SNP.
In 1976, McClure referred that there were characteristic abnormalities suggestive of SNP at CT scan that could help the differential diagnosis of other diseases that affect the nasal fossa and paranasal sinuses.
Many studies referred to CT scan abnormalities found in inflammatory processes (acute or chronic) and tumors in nasal fossa and paranasal sinuses. However, few of them reported CT scan characteristics of SNP.
Drutman et al. (1991, 1993) described two radiological findings considered major findings in the CT scan of SNP, characteristic of the disease: presence of polypoid masses in the nasal cavity (93%, 91%) and widening of infundibulum (89%). This signal has also been found by Babbel & Harnsberger (1991) and Reis et al. (1999).
Minor findings of SNP described by the same authors were: erasing or bone attenuation of ethmoid trabecula (63%) and nasal septum (37%), partial or complete pansinuses opacification (90%), bulging or convexity of papyraceous lamina (51%), water-air levels (43%), and suggestive signals of acute sinusitis (40%). These signals may help the diagnosis when present in the association with one or both major findings. Other authors such as Babbel & Harnsberger (1992) and Yousem (1993) also agreed with these findings.
Liang et al., in 1996, described a new signal related to sinonasal polyposis, "truncation of middle concha bone", observed by them in 58% of the studied patients and advocated as a signal specific for sinonasal polyposis, given that it had not been observed in any other pattern of sinosal inflammatory disease.
This study aims at assessing the agreement level between two observers using CT scan and considering the presence or not of three radiographic signals suggestive of sinonasal polyposis, to wit: 1. widening of ostiomeatal complex; 2. lateral bulging of papyraceous lamina; 3. effacement of bone trabeculum in ethmoid labyrinth.
MATERIAL AND METHODWe consecutively assessed 32 CT scan exams of patients with sinonasal polyposis with histological diagnosis confirmed by the Service of Pathology, Hospital and Maternidade Celso Pierro, PUC Campinas. Patients were followed up by the Service of Otorhinolaryngology in the hospital in the period between January 1997 and June 2000. Eighteen patients (18) were male and fourteen (14) were female subjects. Ages ranged from 12 to 74 years. The mean age was 43 years and median was 47 years. All patients agreed and signed the informed consent term approved by the Ethics Committee of the center.
Inclusion criteria: we included patients examined and treated by the Service of Otorhinolaryngology, Hospital e Maternidade Celso Pierro, PUC-Campinas, who were submitted to anamnesis, ENT physical examination, including anterior rhinoscopy, oroscopy and otoscopy, and clinical pathology confirmation of SNP.
The main complaint reported by patients was nasal obstruction, which made them come to the Service of Otorhinolaryngology.
In the physical examination, we found at least stage II, as provided by the classification proposed by Lund in 19954.
In doubtful cases of presence of polypoid tissue in the middle meatus during the physical examination, patients were submitted to nasal endoscopy with flexible nasofibroscope 3.2mm Machida.
As to polypoid tissue, it was either keratinized or had abnormal color, preoperative incision biopsy was conducted and the material was submitted to the Service of Clinical Pathology, Hospital and Maternidade Celso Pierro, to clarify the diagnosis.
Before conducting the CT scan, patients used cephalexin (500mg QID) for 15 days if presenting rhinorrhea. They were all submitted to nasal lavage with sterile solution and used topical corticoids (mometasone or fluticasone) at least for 15 days before the test.
According to the CT scan staging proposed by Stamm in 199218, all studied patients were at least in stage II, with visible polypoid masses at anterior rhinoscopy, even if still mild.
During the surgical procedure, we collected fragments of the polyps and they were sent to clinical pathology and diagnostic confirmation.
Studied patients were not submitted to histology investigation of polyps concerning the percentage of eosinophil present. We decided not to investigate presence of aspirin intolerance, asthma, or diseases associated with SNP, such as allergic rhinitis and mucoviscidosis.
The patients had not been submitted to any type of nasal or paranasal surgical intervention before.
Exclusion criteria: we excluded from the study patients that had previous undergone surgical treatment, incomplete examinations without both planes referred - axial and coronal sections, or CT scans conducted with inappropriate technique.
We did not enroll in the study patients that had single and unilateral polyps (antrochoanal or sphenochoanal).
Interobserver analysis: in order to check agreement level between observers, CT scans were separately assessed by two radiologists experienced in paranasal sinuses diseases (RPS1 and JLCM2), using a previously defined questionnaire.
Observers responded to the direct questionnaire with simple yes/no questions, assessing the three CT scan signals of SNP, namely: presence or absence of infundibular widening of ostiomeatal complex, presence or absence of lateral bulging of papyraceous lamina, and presence or absence of trabecular bone effacement of ethmoid labyrinth. These signals were chosen because they had been considered the most relevant for the disease.
The radiologists assessed the CT scan exams without exchanging information one with the other and they did not have access to the clinical history of patients.
Equipment, protocol and techniques of exams were: patients were referred to CT scan in the Imaging Diagnosis Unit of Hospital e Maternidade Celso Pierro, PUC Campinas. There was no special preparation of patients immediately before the conduction of the CT scan by the radiology team.
The device used by the service was an axial tomographer brand SYTEC-SRI GE Medical Systems, General Electric Company, Milwaukee, Wisconsin, USA. The test comprised axial and coronal sections.
Coronal sections were made perpendicularly to the hard palate, along the anterior wall of frontal sinus to the posterior wall of sphenoid sinus, showing ostiomeatal complex, correlation between brain and ethmoid roof, orbit and paranasal sinuses and the sphenoethmoid recess.
Axial sections were made parallel to the hard palate, along the floor of the maxillary sinus to the roof of frontal sinus, assessing sphenoid and posterior ethmoid sinuses, defining the correlation between internal carotid arteries and optical nerves with the referred sinuses.
Sections were made at 3 to 5mm thickness and documented in electronic windows devised for assessment of bone and soft tissues (WW 2500-3500).
Intravenous contrast was only used when there were atypical images of polyposis, with presence of extensive bone erosion, or when the description of the ENT physician was doubtful. In such cases, the use of contrast allowed differentiation between aggressive sinonasal polyposis and mucoceles or nasal or sinonasal neoplasm.
CT scans were printed in film brand AGFA, 45cm X 35cm, by laser processor.
Statistical analysis: to assess interobserver agreement level in reading the CT scan exams we used the parametric chi-square test (X2) in contingence table (level of significance of 0.05) and non-parametric tests of Kendall Correlation Coefficient (significance level of 0.05 and 0.01).
RESULTSAnalysis of CT scans of SNP concerning presence of three CT signals was conducted by two radiologists, 1 and 2, and the results are presented in Tables 1 and 2, respectively.
In the statistical study of interobserver agreement the value of chi-square was not significant for CT scan signal of infundibular widening of ostiomeatal complex and lateral bulging of papyraceous lamina (p = 0.7055 and p = 0.2057, respectively), whereas for trabecular bone effacement of ethmoid labyrinth chi-square value was significant (p = 0.0040).
Kendall correlation coefficient between both observers was significant in the three CT scan signals, showing agreement between observers in the assessment patient x patient, even in the effacement signal, in which chi-square was significant.
The main agreement between observers was detected in widening of infundibulum (0.7474) and more frequently in the positive results of the signal.
Frequency of positive results between observers was similar for lateral bulging of papyraceous lamina and for infundibular widening of ostiomeatal complex, closer to the latter. Lateral bulging of papyraceous lamina was less frequently found than infundibulum widening.
Figures 1, 2 and 3 show the CT scan signals studied in SNP.
DISCUSSIONPolyps are the most common expansive lesions of nasal cavity and CT scan is very important in the diagnosis and staging of the disease.
Polyps can be usually identified in the CT scan as discreet diffuse masses of soft tissue with liquid density, owing to intercellular accumulation of liquid inside it and its hypocellular nature. Most polyps tend to present mucoid attenuation with mucosa hypertrophy occasionally seen on the polyp surface.
Drutman et al. (1993) described that one of the radiological findings that distinguish sinonasal polyposis from sporadic isolated polyps and other polypoid lesions such as retention cysts, ethmoid mucoceles, antrochoanal and sphenochoanal polyps is diffuse and bilateral sinonasal nature of the process. There is moderate filling of the lesion when using the contrast.
CT scan signals suggestive of SNP that we studied in this paper comprised infundibular widening of ostiomeatal complex, papyraceous lamina bulging and effacement of trabecular bone of ethmoid labyrinth.
Infundibular widening is an important radiographic criterion in sinonasal polyposis. According to Drutman et al. (1993), this signal was identified in 89% of the patients with sinonasal polyposis studied by him with coronal sections and in 87% of the patients studied by Reis et al. (1999). Som et al. (1991 and 1992), Babbel & Harnsberger (1991), also shared the same finding that infundibular widening is frequently found in sinonasal polyposis; however, they did not report statistical data in their studies.
Similarly to the studied literature, this signal was observed in our study in 84.4% and 90.6% of the cases according to radiologists 1 and 2, respectively. Chi-square was not statistically significant (p = 0.7055) between observers and the agreement between them, measured by Kendall coefficient, was high (R = 0.7474; p = 0.001).
The result, similar to literature reports, made us also state that infundibular widening is an excellent CT scan indicator of sinonasal polyposis, even when polypoid masses are not observed in the nasal fossa with anterior rhinoscopy or endoscopic examination.
However, Drutman et al. (1993) emphasized that infundibular widening is not specific and may be present in cases of antrochoanal polyps, postoperative complications and cases of nasal and sinosal tumors, such as inverted papilloma and mucoceles. This widening can also be found in mycetoma cases.
Nevertheless, we know that in most cases, antrochoanal polyps are unilateral and solitary and not bilateral, as in sinonasal polyposis 19. Most antrochoanal polyps are present in young adults and adolescents, which is also different from the mean age of onset of sinonasal polyposis.
Clinical history of patients can also help us identify whether infundibular widening is secondary to previous surgical procedures; the presence of nasal or paranasal tumors may be manifested by other CT scan findings that support differential diagnosis, such as bone erosion (in tumors and mucoceles), bone sclerosis (chronic processes such as fungi) and heterogeneous aspects (such as in mycetoma).
The second CT scan signal studied was ethmoidal sinus lateral wall bulging (papyraceous lamina).
Once again, Drutman et al., in 1993, referred that bulging of ethmoidal sinus lateral wall (papyraceous lamina) was found in 51% of the patients with sinonasal polyposis studied by them. Reis et al. (1999) found this signal in 30% of the studied cases. Som et al. (1991) and Babbel et al. (1992) detected the presence of papyraceous lamina bulging in their study, but provided no statistical data about incidence of this finding.
In our study, lateral bulging, that is, papyraceous membrane convexity, was present in 34.4% and 50.0% of the cases according to radiologists 1 and 2, respectively. Chi-square was not statistically significant (p = 0.2057) and there was agreement with Kendal correlation coefficient of R = 0.4606 (p = 0.01).
This result, according to the studied literature, shows us that this signal can be a good indicator of polyposis, but it is less significant than infundibular widening of ostiomeatal complex, previously addressed. According to the literature, opacification of ethmoidal sinuses with lateral convexity of ethmoidal wall should raise the suspicion of sinonasal polyposis affecting the sinus.
This CT scan signal, according to Som & Brandwein (1996), allegedly represents bone remodeling owing to the chronic effect of mass of ethmoid polyps. There are normal anatomical variations in the appearance of papyraceous lamina. In most subjects, it is straight and even concave.
This CT scan finding, however, is also non-specific and can be seen in ethmoid mucoceles, and benign or low malignancy sinonasal tumors 19, 20.
Mucoceles results from accumulation of serous fluid in the submucous layer of sinus mucous lining, with retention of secretion and consequent expansion of cavity. There is destruction of adjacent ethmoid labyrinth caused by necrosis pressure on the site 19. The classical ethmoidal mucoceles does not present its internal architecture in CT scan and does not enhance contrast (differently from sinonasal polyposis), in addition to normally being unilateral 10, 16, 20, 21.
As to sinonasal tumors, either benign or low malignancy, according to Som & Brandwein (1996), we should bear in mind that in sinonasal polyposis the individual ethmoid cells are filled with polypoid mucosa and thick mucoid secretion. Persistence of ethmoid septum such as seen in polypoid lesions undoubtedly means there is benign disease. Most of the times, polypoid masses are separated from the adjacent bone by a thin layer of mucoid tissue, which is observed in 20% of the cases with nasal polyposis. This finding allows us to distinguish polyps from tumors through CT scan. Tumors are in direct contact with the bone and can remodel or destroy it.
The third studied CT scan signal of sinonasal polyposis was bone effacement of ethmoid trabecular bone.
Drutman et al. (1993) identified bone effacement of ethmoid trabecular bone in 63% of the CT scans of sinonasal polyposis cases studied by them. Reis et al. (1999) found the same signal in 79% of the cases. Som et al. (1986), Babbel & Harnsberger (1991), Babbel et al. (1992) agreed with the existence of this CT scan signal in sinonasal polyposis, but did not report statistical data.
Our study showed effacement of ethmoid trabecular bone in 90.6% and in 59.4% of the cases according to radiologists 1 and 2, respectively. Chi-square was significant (p=0.0040), but there was agreement between observers based on Kendall correlation coefficient (R = 0.388; p = 0.03).
We observed there was statistically significant difference in the interpretation of this signal between the two observers, despite the agreement confirmed by Kendall coefficient. This fact shows us how difficult it is to interpret this signal, which should be analyzed together with clinical and radiological data. Lack of detection does not exclude the disease, whereas its presence only indicates likelihood of having the disease.
Our data are in agreement with literature reports that stated that it is harder to detect this signal than the other previous ones reported here. The attenuation of ethmoid trabecular bone that can be found in the nasal septum is probably resultant from chronic pressure exerted by polyposis. Ethmoid trabecular bone, and in some cases nasal septum, are delicate structures that can have less than 1mm thickness, which hinders its visualization by CT scan, given the architecture of the sinus region. Absence of normal number and size of ethmoid trabecular bone or nasal septum in an area that has not been surgically manipulated should call our attention to the likelihood of presenting an aggressive inflammatory process such as sinonasal polyposis 17, 20, 22.
Similarly to literature data, we observed that CT scan signal of papyraceous lamina bulging and ethmoid trabecular bone effacement in sinonasal polyposis should be interpreted together with the main CT scan of SNP: infundibular widening.
Such findings can help us identify the possible extension of the disease into the paranasal sinuses.
A patient with clinical history of SNP, presence or nor of polypoid masses upon anterior rhinoscopy and/or nasal endoscopy and presence of infundibular widening in CT scan will certainly have SNP in the region. If there is opacification of ethmoid sinus with bone trabecular effacement and external convexity of lateral walls of opacified ethmoid bone, the patient also probably has polyposis involving this sinus, even when polypoid masses are not visible by CT scan.
CONCLUSIONAccording to the interobserver analysis using CT scan concerning the presence of the studied signals, we concluded that:
1. Infundibular widening of ostiomeatal complex is the most frequent CT scan finding and the one that generated the most agreement between observers;
2. Lateral bulging of papyraceous membrane is a signal that generated high interobserver agreement, but it is less frequent than infundibular widening of ostiomeatal complex;
3. Ethmoid trabecular bone effacement is a CT scan signal that is hard to interpreter, depending on the observer, but it is one further piece of data for the diagnosis of sinonasal polyposis.
REFERENCES1. Drutman J, Babbel RW, Harnsberger HR, Sonkens JW, Braby D. Sinonasal Polyposis. Seminars in Ultrasound, CT, and MR 1991;12:561-74.
2. Small P, Frenkiel S, Black M. Multifactorial etiology of nasal polyps. Ann Allergy 1981; 46:317-20.
3. Kern RA, Schenck HP. Allergy a constant factor in etiology of so-called mucous nasal polyps. J Allergy 1933; 485-95.
4. Lund VJ. Diagnosis and treatment of nasal polyps. BMJ 1995; 311:1411-4.
5. Rogala B, Namyslowski G, Mrowka-Kata K, Gawlik R, Gabriel A. Concentration of s-ICAM-1 in nasal polyps tissue. Med Sci Monit 2000; 6:1109-12.
6. Settipane GA, Klein DE, Settipane RJ. Nasal polyps. State of art. Rhinol Suppl 1991; 11:33-6.
7. Voegels RL, Santoro P, Butugan O, Formigoni LG, Miniti A. Polipose nasal e alergia: existe correlação? Rev Bras ORL 2001; 67(Pt1):220-7.
8. Lurie MH. Cystic fibrosis of the pancreas and nasal mucosa. Ann Meet Am Laryngol Assoc 1959; 35:478-86.
9. Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis: A review of 6.037 patients. J Allergy Clin Immunol 1977; 59:17-21.
10. Drutman J, Harnsberger HR, Babbel RW, Sonkens JW, Braby D. Sinonasal polyposis: investigation by direct coronal CT. Neuroradiology 1993; 36:469-72.
11. Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, Roberts GD. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc [serial online] 1999 Sept [cited 2000 Nov 23]. Available from: URL:
12. Saleh HA, Lund VJ. Treating nasal polyps. Practitioner, 2000; 244:84-93.
13. Hassid S. Sinusonasal polyposis. Acta Otorhinolaryngol Belg 1997; 51:367-70.
14. Guimarães RES, Becker HMG. Epidemiologia da polipose nasal. In: Stamm AC, editor. Rhinologia 2000. São Paulo: Revinter; 2000. p. 203-6.
15. Zinreich SJ, Kennedy DW, Rosenbaum AE, Gayler BW, Kumar AJ, Stammberger H. Paranasal sinuses: CT imaging requirements for endoscopic surgery. Radiology 1987; 163:769-75.
16. Forbs WSTC, Fawcitt RA, Isherwood I, Webb R, Farrington T. Computed Tomography in the diagnosis of the paranasal sinuses. Clin Radiol Manchester 1999; 29:501-11.
17. Brihaye P, Clement PAR, Dap I, Desprechin B. Pathological changes of the lateral nasal wall in patients with cystic fibrosis (mucoviscidosis). Int J Ped Othorhinolaryngol 1994; 28:141-7.
18. Stamm AC. editor. A surgical staging system for sinonasal polyposis. XVIII Pan American Congress of ENT. Head and Neck Surgery 1992 Orlando, USA; p.115.
19. Som P, Brandwein M. Sinonasal Cavities: Inflammatory Diseases, Tumors, Fractures, and Postoperative Findings. In: Som P, editor. Midface and sinonasal cavities; 1996. p. 142-85.
20. Finn DG, Hudson WR, Baylin G. Unilateral polyposis and mucoceles in children. Laryngoscope 1981; 91:1444-9.
21. Som PM, Lawson W, Biller HF, Lanzieri CF. Ethmoid Sinus Disease: CT Evaluation in 400 Cases. Radiology 1986; 159:591-7.
22. Som PM, Sacher M, Lawson W, Biller HF. CT Appearance Distinguishing benign nasal polyps from malignancies. J Comp Ass Tomogr 1987; 11:129-33.
23. Babbel RW, Harnsberger HR, Sonkens J, Hunt S. Recurring patterns of inflammatory sinonasal disease demonstrated on screening sinus CT. AJNR 1992; 13:903-12.
24. Babbel RW, Harnsberger HR. A contemporary look at the imaging issues of sinusitis: sinonasal anatomy, physiology, and computed tomography techniques. Seminars in Ultrasound, CT, MR 1991; 12:526-40.
25. Liang EY, Lam WW, Woo JK, Van-Hasselt CA, Metreweli C. Another CT sign of sinonasal polyposis: truncation of bony middle turbinate. Eur Radiol 1996; 6:553-6.
26. Lund VJ, Mackay V. Staging in rhinosinusitis. Rhinology 1993; 107:183-4.
27. McClure W. Chronic Hypertrophic Polypoid Rhinosinusitis. Radiology 1976; 120:609-13.
28. Reis CMCB, Marchiori E, Pereira CIGS, Apa EAP. Polipose sinonasal. avaliação por tomografia computadorizada. Revista da Imagem 1999; 21:101-6.
29. Som PM, Lawson W, Lidov MW. Simulated aggressive skull base erosion in response to benign sinonasal disease. Radiology 1991; 180:755-9.
30. Yousem DM. Imaging of sinonasal inflammatory disease. Radiology 1993; 188:303-14.
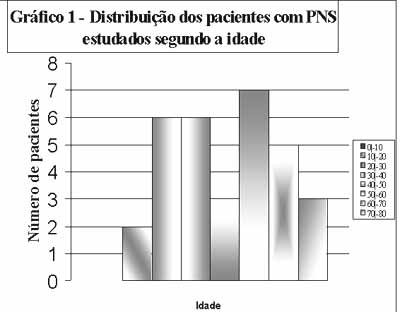 Graph 1.
Graph 1. Distribution of patients with SNP according to age.
Table 1. Assessment of 32 CT scans of SNP according to radiologist 1.
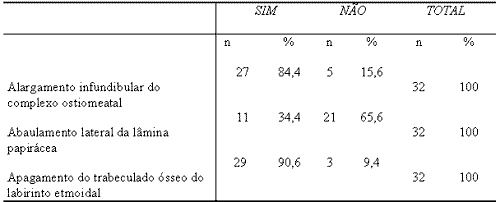
n=Number of patients
% = percentage of patients
Table 2. Assessment of 32 CT scans of SNP according to radiologist 2.
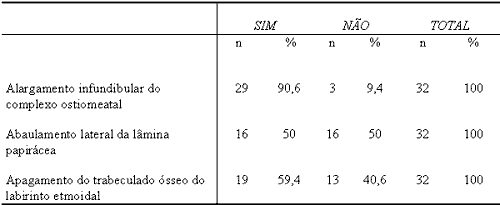
n=Number of patients
% = percentage of patients
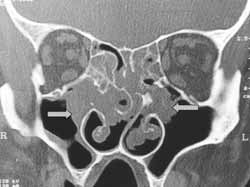 Figure 1.
Figure 1. CT scan of infundibular widening of ostiomeatal complex at coronal section (case 13).
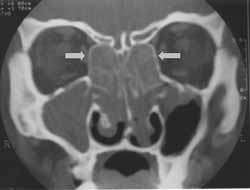 Figure 2.
Figure 2. CT scan of lateral bulging of papyraceous membrane at coronal section (case 1).
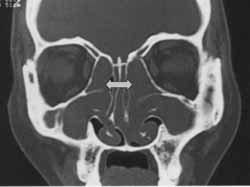 Figure 3.
Figure 3. CT scan of bone effacement of ethmoid trabecular bone at coronal section (case 31).


