INTRODUCTIONSystemic lupus erythematosus (SLE) is a systemic chronic multifactorial inflammatory disease of unknown etiology that progresses with episodes of activity and episodes of remission. It is immunologically characterized by presence of multiple autoantibodies, being that clinical manifestations are very polymorphic. Tissue damage occurs owing to specific autoantibodies or deposition of antigen-antibody complexes. There may be impairment of osteo-articular, muscle, mucous-cutaneous, vascular, renal, nervous, heart, lung, gastrointestinal, hematological, ocular and auditory systems 1.
The inner ear can be affected by different mechanisms, being that the most frequent manifestation is bilateral fluctuating sensorineural loss, quickly progressive and with response to immunosuppressants 2.
Sensorineural loss of autoimmune etiology does not have precise estimates: in 65% of the cases it affects women aged 17 to 42 years 3-5. Early diagnosis is essential since it is one of the few reversible causes of sensorineural loss, in which early treatment can prevent auditory degeneration or even recovery in some cases.
The purpose of the present study was to discuss the possible mechanisms involved in affecting the inner ear by immune-mediated diseases, as well as to correlate the role of chloroquine (drug used in the treatment of SLE) as maximizing the hearing loss.
LITERATURE REVIEWThe literature 6, 7 has few cases described in which sensorineural loss was attributed to SLE, ruling out any other cause 8. The inner ear can be the site of organic-specific diseases or, more commonly, be present in a disease that has a systemic form. This autoimmune mechanism is capable of triggering sensorineural loss by self-damage to the inner ear 5.
Experimental studies demonstrated that the inner ear is an immunocompetent organ, capable of presenting humoral immune response before local or systemic antigen stimuli, being that the endolymphatic sac is the region that has more potential to immune processing 2-4.
In a study conducted by Andronopoulos 8 involving 40 patients with SLE, auditory loss was not related to duration or disease activity, nor with renal involvement or central nervous system, treatment used, antibody level or complement.
The main pathophysiological mechanisms of autoimmune character involved in inner ear dysfunction include: immediate hypersensitivity, with production of IgE immunoglobulins against cochlear antigens, deposits of immunocomplexes in vascular stria and spiral ligament, direct action of T cell cytotoxic in cochlea and late hypersensitivity causing immune reactivity mediated by type II collagen fibers. These mechanisms can act in a complementary fashion and many times simultaneously 3, 5. It is known that the main histopathological findings are spiral ganglion degeneration, Corti's organ atrophy, vascular stria degeneration, precipitation and atrophy of endolymphatic sac, presence of macrophages and precipitates of endolymph, in addition to perivascular infiltrate and deposition of circulating immunocomplexes in vascular stria 4, 9.
In a study conducted in 1999 by Michihiko10, the main histopathological alterations found in 14 temporal bones of patients with SLE were damage to spiral ganglion cells, with varying grade of hair cells and vascular stria atrophy. They also found tissue and bone fibrosis in the cochlea with total loss of membranous labyrinth. Cochlear hydropsis was found in one ear.
Initially, vasculitis secondary to SLE was advocated as being the basic mechanism in pathogenesis of sensorineural loss. Caldarelli et al.4 suggested a mechanism of microinfarction of capillaries and arterioles in the temporal bones. Hisashi et al.11 were the first ones to indicate the association between sensorineural loss in patients with lupus and the presence of anti-phospholipid antibody, suggesting that these antibodies induced to thrombosis of the inner ear region.
Antiphospholipid antibody syndrome (AAS) is a disorder in which there may be venous and/or arterial thrombosis recurring in different sites, such as central nervous system, retina, heart, lung, liver, kidneys and ears. Repetitive miscarriages are frequent, associated with thrombocytopenia. Anticoagulant and/or positive antibodies anticardiolipin in moderate or high levels can also be found 10, 12, 13.
Audiovestibular impairment may occur owing to vascular lesions to the internal auditory artery, which can take to cochlear and vestibular symptoms, both isolated or associated.
Clinical picture of immune-mediated hearing loss is sudden or quickly progressive, fluctuating sensorineural hearing loss that occurs in weeks or months, asymmetrical and bilateral in most cases (79%). In approximately one third of the cases it is not associated with vestibular symptoms, since gradual affection of the system promotes labyrinthic compensation 3-5, 8.
Studies demonstrated that 25 to 50% of the cases simulate hydropsis, but non-characterization of the classical triad (tinnitus, vertigo and hearing loss) as well as quick progression of bilateral hearing loss speaks in favor of the diagnosis of autoimmune inner ear disease.
It is known that 25 to 50% of the cases of hydropsis can present only cochlear symptoms. Studies suggested that deposition of immunocomplexes on the vascular stria or endolymphatic sac interfere in the production and absorption of endolymph, resulting in endolymphatic hydropsis 8.
In 29% of the cases hearing loss exists together with systemic manifestations of the autoimmune disease. Normally ENT exam does not present alterations and otoneurological test may have reduced responses 5, 8, 11, 14.
Audiometry shows curves that may present ascending or descending format, symmetrical or asymmetrical. The percentage of speech recognition index presents unpredictable behavior, not proportional to pure tone thresholds. Immittanciometry can present normal tympanometric curve and acoustic reflexes 5.
Aiming at diagnosing, data that suggest the disease are bilateral rapidly progressive and inexplicable hearing loss, onset at middle ear, higher incidence in female subjects, normal otoscopy, and systemic disease of associated immune etiology. In lab tests, we can conduct nonspecific lab tests, such as inflammatory activity tests, rheumatoid factor, antinuclear factor, circulating immunocomplex, which demonstrate the activity of the disease, serum immunoglobulin dosages and production of autoantibodies such as type II collagen anti-collagen 15, 16. The frequency of antiphospholipid antibodies in patients with SLE is not well determined, and there is great variation in the literature (18 to 86%), owing to the use of different measurement methods and fluctuations of levels of antibodies found. Toubi et al. 12 found 20% positivity in 100 patients with SLE without manifestations of central nervous system or thromboembolic. Frequency goes up to 50% when there is central nervous system manifestation. Specific tests can be conducted including lymphocytarian transformation, inhibition of lymphocytarian migration, ELISA and immunofluorescence against inner ear antigens 5, 3, 11, 17. Another test that can be conducted is anti-antigen antibody 68 KD. Positivity is indicative of good responsiveness to corticoid therapy with sensitivity of 42% and specificity reaching 90% 4, 5.
Magnetic resonance imaging with contrast (gadolinium) in T1 can demonstrate inflammatory activity in membranous labyrinth.
According to clinical findings and complementary tests conducted, differential diagnosis should be made with other disease, such as tumors, infections, ototoxicity or hereditary auditory losses 3.
Ototoxicity of antimalaria agents frequently employed in treatment of SLE has been known for some time, but it has not been much communicated. Hearing loss caused by these drugs may be reversible or irreversible, as a result of duration of administration 18-21. In the study by Andronopoulos 8, hearing loss was not attributed to ototoxic effect of hydroxychloroquine, but it is reported in the literature that antimalaria agents cause destruction of inner and outer hair cells 18.
Normally, corticoid therapy with prednisone (1mg/Kg/day) is the first treatment option in autoimmune disease, and in some cases clinical improvement during treatment is a diagnostic criterion. In non-responsive cases, immunosuppression (cytotoxic drugs such as methotrexate and cyclophosphamide) can be used and in case of failure, plasmapheresis can be employed as complementary therapy 2, 3, 5. In patients with syndrome of antiphospholipid antibody, the use of anti-coagulation should be considered 12. In general, in rapidly progressive hearing loss of unknown cause in young patients, we should consider the hypothesis of autoimmune etiology. However, serum levels indicative of positive exams are rarely reached, hindering the diagnosis of systemic disease.
CASE REPORTCase 1
L.S., female, 24-year-old, Black-descendent, born and living in Santo Andre (SP), came to the ambulatory of Otorhinolaryngology, FMABC, with history of bilateral fluctuating hearing loss, quickly progressive for 2 years, especially on the left, followed by occasional endotic tinnitus. She did not report dizziness and family history of hearing loss. Two years and 8 months before, the diagnosis of SLE was made, following the criteria of the American College of Rheumatology, and of secondary Sjögren's syndrome. She started treatment with antimalaria agent (chloroquine diphosphate) and prednisone low dose.
The patient was under control of the systemic disease, but maintained the complaint of hearing loss and was referred to the Discipline of Otorhinolaryngology.
Physical ENT examination showed no affections.
We ordered serial audiometric tests (Figures 1 to 3), inflammatory activity tests, total and fractions of complement, rheumatoid factor, FAN, total blood count, evoked auditory potentials, otoacoustic emissions, and imaging tests (CT scan and MRI).
The patient presented positive FAN, blood count that showed leucopoenia and serial audiometry with fluctuating, asymmetrical, progressive hearing loss, speech recognition index not proportional to pure tone thresholds.
Distortion product otoacoustic emissions were absent on the right and present on the left. Imaging tests were normal.
Owing to the possibility of having autoimmune inner ear affection, we decided to increase the corticoid dose and observed slight improvement of audiometric thresholds on the left ear. We decided to suspend the antimalaria agent after talking to the Rheumatology department, since the possible ototoxic effects could maximize the auditory impairment of the patient, being a contributing factor to auditory deterioration. We introduced methotrexate with clinical monitoring and monthly audiological assessments, but there was no satisfactory improvement of the clinical picture.
Case 2
A. O. C., female, 41-year-old Caucasian patient, born and living in Sao Paulo, with history of fluctuating bilateral hearing loss for 1 year, especially on the right, followed by intermittent tinnitus on the same side and occasional non-rotation dizziness. She did not report otorrhea and otalgia. Normal ENT examination.
Eight years before she had been diagnosed with SLE, being initially treated in another service with antimalaria agents and high doses of corticoids. She quit treatment because she had gained weight. One year before, she restarted treatment at the Ambulatory of Rheumatology, Medical School, ABC, where she was instructed to reintroduce antimalaria agent.
We ordered audiometry that presented bilateral, asymmetrical sensorineural loss with normal speech recognition index. In a new test 3 months after treatment with corticoids, there was no improvement in auditory thresholds (Figure 4).
Case 3
N. F. G., female, 45-year-old Caucasian patient, born in Santo André, with history of sudden deafness on the left 3 months before, followed by tinnitus and no other associated factors. The ENT examination did not present any abnormality.
The patient had SLE and had been submitted to treatment with antimalaria agents for 3 years. We conducted audiometry (Figures 5 and 6) that showed severe sensorineural loss with ascending audiometric configuration on the left and very low speech recognition index on the same side (40%). Tulio phenomenon was absent bilaterally. MRI was normal and evoked auditory potential did not present signs of retrocochlear affection. We started therapy with prednisone at 60mg/day dose, later regression for 15 days and improvement of audiological condition.
DISCUSSIONThe cases presented were received in our service with diagnosis of SLE and controlled systemic disease as a result of the proposed treatment, except for the presence of progressive and fluctuating hearing loss.
In the first two cases described, there was quick evolution of the hearing loss and in the third case, the patient presented sudden hearing loss. This sudden character could be in agreement with vascular lesions in the internal auditory artery, such as antiphospholipid antibody syndrome. In this case, the lipid anticoagulant dose and anticardiolipin antibody could help in the identification of these thromboembolic phenomena. However, positivity of these cases reach only 20% 11. The risk of anticoagulation as the treatment of choice would not justify the benefits for the patient. Another factor that increases the possibility to thromboembolic phenomena in the inner ear is absence of inflammatory activity in magnetic resonance, conducted early in this case.
As to diagnostic tests, all patients showed affections in nonspecific exams to autoimmune diseases. Unfortunately, the public healthcare system in Brazil does not have anti-68KD available, which is the most specific test in cases of autoimmune hearing loss, reason why it was not performed in the patients.
Initial treatment performed in the three cases was corticoid therapy with prednisone in high dosage (1mg/Kg/day) for 20 to 30 days. We only had improvement of the third patient, according to audiometric criteria (improvement in pure tone average of 15dB HL, 20% in vocal discrimination or hearing stabilization).
In all cases, ototoxicity of chloroquine was object of discussion. Hearing loss caused by this drug can be reversible if it is suspended at initial stage of inner ear impairment 18. However, chloroquine deposited in tissues takes on average two months to be completely eliminated from the body, therefore, the action still persists after its suspension.
Initial lesions can be detected by pure tone and vocal audiometry, monitoring of evoked auditory potentials and otoacoustic emissions 21.
Therefore, there are no doubts about inner ear impairment by SLE and other immuno-mediated disease, reason why the possibility of associating factors (immune, vascular and ototoxic) contributing to the auditory worsening of the patient should also be investigated. However, this diagnosis was very difficult to be defined in the reported cases. When the audiological investigation started, the patient had already been submitted to the ototoxic effect of the antimalaria agent, being difficult to assess the level of damage to hearing that the drug could have caused 18, 20. In our first patient, we tried to change chloroquine for immunosuppressant therapy with methotrexate. However, maybe because of the high level of hearing loss, we did not succeed.
Another important factor to be considered is the risk-benefit ratio to the patient, since if there is systemic control of the disease with administration of antimalaria agent, the use of immunosuppressant to replace it may lead to other significant adverse effects.
CLOSING REMARKSIn the present study we observed that sensorineural loss can be present in subjects with immune-mediated systemic diseases, which can affect the inner ear owing to different mechanisms. We suggest that as soon as the systemic disease is diagnosed the patient, if asymptomatic, should be referred to audiological investigation, preventing future damage.
The opposite is also true: in view of a patient with progressive or sudden fluctuating sensorineural loss, we should consider the investigation of systemic diseases by the appropriate department, in an attempt to explain the hearing loss and even try to reverse it in some cases.
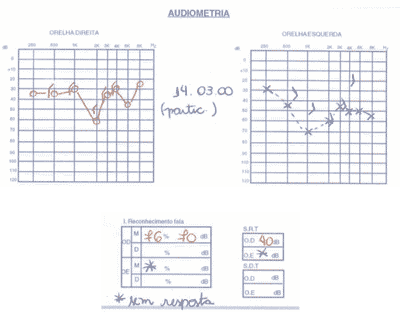
Figure 1. Pacient 1
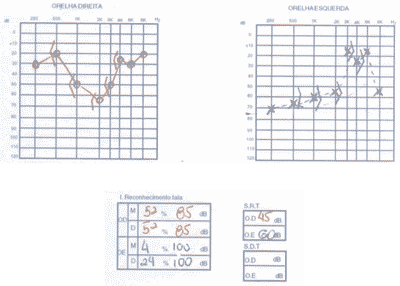
Figure 2. Pacient 1
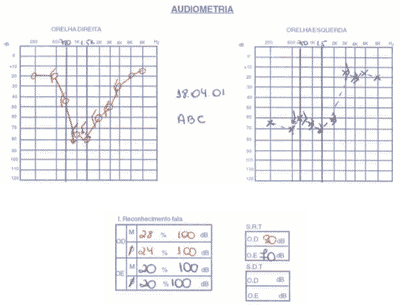
Figure 3: Pacient 1
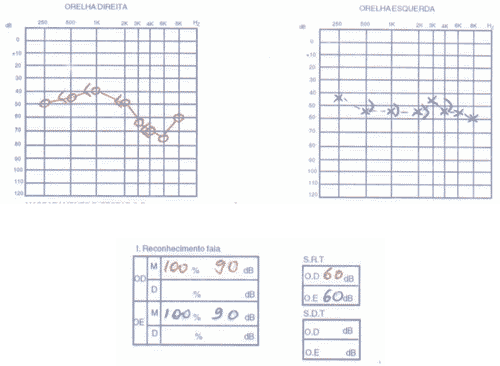
Figure 4. Pacient 2
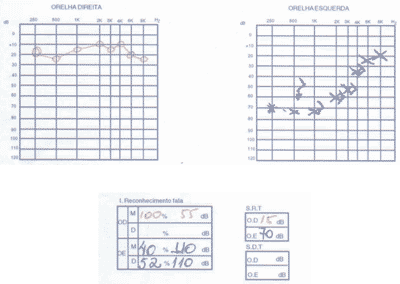
Figure 5. Pacient 3
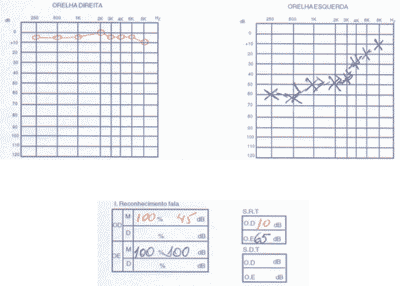
Figure 6. Pacient 3
1. Sato EI. Lupus Eritematoso Sistêmico. Diagnóstico e Tratamento Atual de Doenças Reumáticas 1999; 10:2-16.
2. Harris JP. Immunologic Disorders Affecting the Ear In: Cummings C. Otolaryngology. Head and Neck Surgery. United States 1999: 3172-85.
3. Decoster DMH, Ferreira NGM, Marques MPC. Doenças auto-imunes da orelha interna. uma revisão bibliográfica. Acta Awho 2001; 20(2):113-16.
4. Bitar RSM, Thomé DC, Nascimento EV, Sanchez TG. Doenças auto-imunes da orelha interna: Revisão da literatura. Arquivos da Fundação Otorrinolaringologia 1998; 2: 92-9.
5. Cruz OLM, Costa SS, Alvarenga EL. Disacusia Neurossensorial Imunomediada In:.Cruz OLM, Costa SS. Otologia clínica e cirúrgica. Rio de Janeiro: Revinter; 2000. p. 307-13.
6. Hamblin TJ, Mufti GJ, Bracewell A. Severe deafness in SLE: its immediate relief by plasma exchange. Br Med J 1982; 284:1374-5.
7. Caldarelli DD, Rejowski JE, Corey JP. Sensorineural hearing loss in lupus erythematosus. Am J Otol 1986; 7:210-3.
8. Andronopoulos AP, Naxakis S, Goumas P, Lygatsikas C. Sensorineural hearing disorders in systemic lupus erythematosus: a controlled study. Clin Exp Rheumatol 1995; 13:137-41.
9. Yoon TH, Paparella MM, Schachern PA. Systemic Vasculitis: A Temporal Bone Histopathologic Study. Laryngoscope 1989; 99:600-9.
10. Sone M, Paparella MM, Schachern PA, Morizono N. Study of Systemic Lupus Erythematosus in Temporal Bones. Ann Otol Rhinol Laryngol 1999; 108: 338-44.
11. Hisashi K, Komune S, Taira T, Uemura T, Sadoshima S, Tsuda H. Anticardiolipin Antibody-Induced Sudden Profound Sensorineural Hearing Loss. Am J Otolaryngol 1993; 14:275-7.
12. Naarendorp M, Spiera H. Sudden Sensorineural Hearing Loss in Patients with Systemic Erythematosus or Lupus-like Syndromes and Antiphospholipid Antibodies. J Rheumatol 1998; 25:589-92.
13. Vyse T, Luxon M, Walport MJ. Audiovestibular manifestations of the antiphospholipid syndrome. J Laryngol Otol 1994; 108:57-9.
14. Hughes GB, Kinney SE, Barna BP, Calabrese LH. Practical versus theoretical management of autoimmune inner ear disease. Laryngoscope 1984; 94:758-67.
15. Hughes GB, Kinney SE, Barna BP, Calabrese LH. Autoimmune inner ear disease: laboratory tests and audio-vestibular treatment responses. In: Veldman JE, McCabe BF. Oto-immunology. Amsterdam: Kugler Publ; 1987. p.149-155.
16. Yoo TJ, Floyd R, Ishibe T, Shea JJ, Bowman C. Immunologic testing of certain ear diseases. Am J Oto 1985; 6: 96-100.
17. Veldman JE. Immune-mediated inner ear disorders. New syndromes and their etiopathogenesis. In: Veldman JE, McCabe BF. Oto-immunology. Amsterdam: Kugler Publ; 1987. p.125-42.
18. Johansen PB, Gran JT. Ototoxicity due to hydroxychloroquine: Report of two cases. Clin Exp Rheumatol 1998; 16:472-4.
19. Dwivedi GS, Mehra YN. Ototoxicity of chloroquine phosphate. J Laryngol Otol 1978; 92: 701-3.
20. Matz GJ, Nauton RF. Ototoxicity of chloroquine. Arch Otolaryngol 1968; 88:370-2.
21. Bernard P. Alterations of Auditory Evoked Potentials during the Course of Chloroquine Treatment. Acta Otolaryngol (Stockh) 1985; 99:387-92.
22. Xenelli SJ, Morrison AW, Mcclowskey D, Festenstein H, Path FRC. HLA antigens in the pathogenesis of Ménière´s disease. J Laryngol Otol 1986; 100:21-24.
1 Resident Physicians, Service of Otorhinolaryngology, Medical School, ABC.
2 Teaching Assistant, Service of Rheumatology, Medical School, ABC.
3 Professor, Course of Speech and Hearing Pathology, Universidade Metodista de Sao Paulo.
4 Faculty Professor, Service of Otorhinolaryngology, Medical School, ABC.
Affiliation: Medical School, ABC and Hospital Estadual Santo André - Disciplines of Otorhinolaryngology and Rheumatology.
Address correspondence to: Suzana Boltes Cecatto - Rua Sao Paulo, 2484 Bairro Barcelona 09541-100 Sao Caetano do Sul, Sao Paulo
E-mail: suzanacecatto@yahoo.com.br
Study presented at 36º Congresso Brasileiro de Otorrinolaringologia


