INTRODUCTIONAutoimmune sensorineural hearing loss is one of the few causes of hearing loss in which early treatment can prevent auditory degeneration and even recover it in many cases. It is classified as a form of late acquired (non-genetic) hearing loss 1 and it is manifested as sensorineural, fluctuating, bilateral, rapidly progressive hearing loss responsive to treatment with immunosuppressant drugs 2. Approximately 65% of the cases occur in women aged 20 to 40 years, being extremely rare in the pediatric population 3. In this study, the authors aimed at describing a case of sensorineural loss in a child aged 7 years and demonstrate that even though it is a well-defined disease, diagnosis and treatment are still a challenge, especially in children.
CASE REPORTP.F.S.J., male 7-year-old patient, referred to the Service of Otorhinolaryngology, Medical School, ABC, complaining of bilateral hearing loss, worse on the right ear for 2 years, insidious onset, progressive and with periods of fluctuation perceived by the mother and also by the patient. He did not present nasal or oropharynx complaints. The mother did not report complications during pregnancy, delivery and post-delivery. She did not report use of ototoxic drugs during pregnancy, parents' consanguinity, as well as family history of hearing loss.
The patient presented history of viral meningitis, which had occurred 5 years before without complications or sequelae and he did not report hearing loss at the time.
ENT physical examination was normal.
The first audiometric exam revealed moderate sensorineural hearing loss on the left in low and medium frequencies (mean of 33 dB) and profound mixed hearing loss on the right ear. Acoustic immittance presented type A curve bilaterally and absence of right ear reflexes (Figure 1).
We conducted serial audiometry with a 2-month interval in an attempt to assess fluctuation of hearing reported by the mother. We noticed that the left ear presented slight pure tone thresholds improvement (mild sensorineural loss with average of 20dB), and on the right side there was hearing worsening, presenting practically no response (Figure 2).
We ordered serology for cytomegalovirus, toxoplasmosis, rubella and syphilis, and rheumatic tests - erythrocyte sedimentation rate, rheumatoid factor, reactive protein C and antinuclear factor. Serology was negative and only sedimentation rate and reactive protein C were above normal range.
Computed tomography did not reveal any abnormalities.
We considered the hypothesis of autoimmune hearing loss and started corticoid as trial therapy. Initial dose was 20mg prednisolone daily divided into 2 intakes.
Audiometric control was made after 1 month of treatment and showed significant improvement in pure tone thresholds, especially on the left ear, which practically reached normal range (mean of 7dB ) (Figure 3).
The mother and the patient reported significant hearing improvement, but 6Kg increase in weight.
We started to reduce the dose of the drug to have safe maintenance dose for 3 months and keep hearing thresholds, without bringing up the undesirable side effects of corticoid therapy. The dose was gradually reduced and currently (ten months after onset of treatment), the patients takes 2.0mg prednisolone daily and has maintained unaltered thresholds.
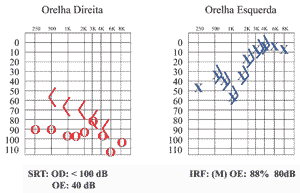
Figure 1. Pure tone audiometry conducted in the first visit.
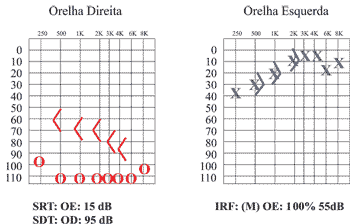
Figure 2. Audiometry
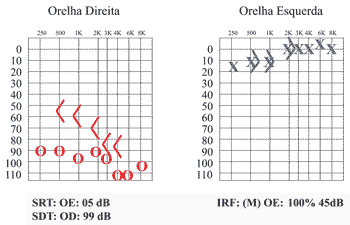
Figure 3. Pure tone audiometry one month after corticoid use.
Autoimmune disease is characterized by reaction of the immune system against autologous antigens, which should be recognized as owned by the body, providing tissue lesion. It in general results from failure or rupture of mechanisms that normally are responsible for maintenance of self-tolerance 4.
The diagnostic landmark is rapidly progressive sensorineural loss that occurs in a period of weeks to months, frequently bilateral and asymmetrical. It may be associated with vestibular symptoms (vertigo or instability), ear fullness, tinnitus or hearing loss that is sudden or fluctuating. As time goes by, hearing continues to decrease up to total deterioration 5.
Hearing loss can start at any age, being more common in women aged between 30 and 45 years 5.
ENT examination does not reveal abnormalities.
Pure tone audiometric curves are not typical, and they may present ascending or descending shapes. Vocal discrimination also has unpredictable manifestations 7.
Electronystagmography normally presents reduced responses 8.
Diagnosis should be based on clinical course, in laboratory tests and therapeutic response. Laboratory investigation can be made by non-specific tests (circulating immunocomplexes, complement system, total hemolytic complement, circulating immunoglobulin, erythrocyte sedimentation rate, reactive protein C, rheumatoid factor, antinuclear factor, anti-collagen II antibodies, Epstein-Barr virus antibodies, cytomegalovirus, hepatitis B virus, toxoplasmosis and syphilis) and specific tests (lymphocytarian transformation, inhibition of lymphocytarian migration, immunoenzymatic analysis, and immunofluorescence against ear antigens - protein 68kD) 5.
Magnetic resonance imaging allows identification of inflammatory activity in the membranous labyrinth by increase in signal after infusion of paramagnetic contrast (gadolinium) at T1 1.
Corticoid therapy is still the main treatment option for autoimmune sensorineural loss. Prednisone at initial dosage of 1mg/kg/d, up to total dose of 60 mg/d is the first choice. Dexamethasone with dose of 0.1mg/kg/d, up to 8 mg/d or deflazacort with total dose of about 30 mg/d can be alternative options to prednisone.
Duration of treatment depends of the evolution of the case. Normally after 4 weeks it reaches stabilization of the clinical picture, allowing gradual reduction of the drug up to dose of maintenance of about 10 to 20mg/d of prednisone. This dosage should be maintained for at least 3 months. If we observe flaring up of hearing loss or other symptoms, dose increase is indicated 9.
During administration of corticoid, we should monitor the side effects of corticoid therapy and cost-benefit ratio should be widely discussed with the patients and family members.
Indicative criteria of response to corticoid therapy are: 1) improvement in pure tone mean in 15dB or more; 2) increase in vocal discrimination in 20% or more, and 3) stabilization of hearing with complete resolution of vertigo 2.
If we do not get satisfactory response using corticoids, and in more severe cases, we can indicate immunosuppression with cyclophosphamide 1mg/Kg/d for up to 3 months with replacement for prednisone in maintenance dose. We should control the impact of cyclophosphamide in the white series. Leucopoenia below 2,000 cell/ml or neutropenia below 1,000 cells/mm, which can determine the shortening of treatment or even its suspension. Cyclophosphamide can cause hemorrhagic cystitis, urinary tract malignant diseases and nephrotoxicity. Azathioprine and methotrexate have already been proposed in these conditions and also for the treatment of children, for being safe in this age range.
In some cases, with high titers of circulating immunocomplexes and autoantibodies, plasmapheresis can be considered as complementary approach to corticoid or immunosuppressant use 1.
Hughes et al. observed that hearing loss can start at any age, being more common between 30 and 45 years and extremely rare in children 6. Prevalence of disease in this age range varies a lot (from 4 to 30%)10 and some authors suggested that the autoimmune mechanism was much more involved than it had been thought 3.
Owing to difficulty in conducting specific laboratory tests, the definition of precise diagnosis of this disease is hindered and treatment is many times delayed. Dinces et al. reported in their study that the lymphocytarian transformation test is an exam used to diagnose autoimmune hearing disorder with specificity of 93% and sensitivity of 96% 11. Other authors argued that sensitivity is lower and that protein 68kD has similar specificity to lymphocytarian transformation and higher sensitivity. Harris and Hughes considered audiometric improvement with corticoids as a diagnostic method. However, in children there is some resistance to using therapeutic trials, especially by the parents, owing to the many side effects that long-term corticoid use may cause 12.
Initial treatment produces significant improvement in audiometric thresholds, but owing to disease instability, there may be gradual and continuous decrease of auditory function 9.
CLOSING REMARKSAutoimmune sensorineural loss is a pathology normally described in adults, especially in middle-aged women, and few cases have been reported in children. In this report, the patient showed clinical presentation suggestive of hearing fluctuation and elevated erythrocyte sedimentation rate and reactive protein C. However, owing to the impossibility of conducting specific tests for the diagnosis of the disease, we conducted therapeutic trial with corticoids and obtained audiometric improvement. Thus, we confirmed the diagnosis of autoimmune sensorineural loss in a child, and so far he has maintained the same audiological pattern.
Therefore, the authors concluded that we should take into account the possibility of sensorineural hearing loss in all age ranges, including the pediatric population, bearing in mind that the use of corticoids is still the best treatment option and also that they can be used as therapeutic trial when there are no other tests available or when they show negative results.
REFERENCES1. Cruz OLM, Costa SS, Alvarenga EL. Disacusia Neurossensorial Imunomediada. In: Cruz OLM, Costa SS. Otologia clínica e cirúrgica. Rio de Janeiro: Revinter; 2000. p. 307-13.
2. Decoster DMH, Ferreira NGM, Marques MPC. Doenças autoimunes da orelha interna - uma revisão bibliográfica. Acta Awho 2001; 20(2):113-16.
3. Brookhouser PE. Nongenetic sensorineural hearing loss in children. In: Canalis RF, Lambert PR (eds.). The Ear: Comprehensive Otology. Philadelphia: Lippincott Willians & Winkins 2000; p. 489-510.
4. Abbas AK, Lichtman AH, Pober JS. Diseases caused by humoral and cell-mediated immune reactions. In: Cellular and molecular immunology. 1st. ed. Philadelphia: Saunders; 1991. p. 369-76.
5. Bittar RSM, Thomé DC, Nascimento EV, Sanches TG. Doenças auto-imunes da orelha interna: Revisão da literatura. Arquivos da Fundação Otorrinolaringologia 1998; 2:92-9.
6. Hughes GB, Kinney SE, Barna BP, Calabrese LH. Autoimmune inner ear disease: 1990 report. In: The Meeting of The American Otological Society. Palm Beach, Florida; 1990.
7. McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol 1979; 88:585-9.
8. Hughes GB, Kinney SE, Barna BP, Calabrese LH. Autoimmune inner ear disease - laboratory tests and audio-vestibular treatment responses. In: Veldman JE, McCabe BF. Oto-immunology. Amsterdam: Kugler Plu; 1987. p. 149-55.
9. Harris JP. Immunologic Disorders Affecting the ear. In: Cummings C. Otolaryngology Head and Neck Surgery. United States; 1999. p. 3172-85.
10. Berrettini S, Ravecca F, Sellari-Franceschini S, Matteucci F, Siciliano G, Ursino F. Progressive sensorineural hearing loss in childhood. Pediatr Neurol 1990; 20:130-6.
11. Dinces EA, Yang SAB, Balogun AO. Pediatric fluctuating sensorineural Hearing Loss: Problems in medical management. In: Laryngoscope 2001; 111(1): 21-5.
12. Harris JP, Moscicki RA, Hughes GB. Immunologic disorders of the inner ear. In: Hughes GB. Pensac NL eds. Clinical Otology. 2nd ed. New York: Thieme Medical Publishers; 1997. p.381-91.
1 Resident physician, Discipline of Otorhinolaryngology, Medical School, ABC.
2 Full Professor, Discipline of Otorhinolaryngology, Medical School ABC.
3 Resident physician, Discipline of Otorhinolaryngology, Medical School, ABC.
Address correspondence to: R. Sao Paulo Antigo, 319 ap. 51 Real Parque, Sao Paulo SP 05684-010
E-mail: katia.guga@originet.com.br


