INTRODUCTION AND LITERATURE REVIEWIn order to maintain good mouth health, we depend on a number of factors and on appropriate salivary flow. Saliva is a fluid produced by salivary glands that comprises cell debris, bacteria, proteins and food debris. Thanks to its properties of interfering in tissue scaring, act in acid buffering, in dental demineralization and remineralization, being bactericidal and bacteriostactic, and source of growth factors, we can maintain the balance and health of our mouth cavity 1, 2, 3.
Loss of any portion of the salivary gland by obstruction, trauma, removal of tumor tissue or any other pathological abnormality can lead to damage to salivary flow, bringing harmful consequences to the body. Therefore, the deepest knowledge of all involved processes in recovering the affected organ becomes essential.
The development of all salivary glands is similar. The submandibular gland (SMG) in rats, which is an excellent animal model to study the processes that involve organogenesis, starts development at 14 days of intrauterine life and it is completed with differentiation of the acinar compartment at about the 4th week after birth 4, 5. In gland development, which influences both morphogenesis (branched architecture) and cytodifferentiation (expansion of secretive capability of cells), the interactions between ectomesenchyma and epithelial cells are well-defined 6, 7.
However, there are few references in the literature about the regenerative processes of salivary glands submitted to mechanical damage or that had any part of its structure excised.
Based on these facts, the purpose of the present study was to better understand the process of regeneration of SMG in rats submitted to partial excision of one of the lobes.
We used staining technique with hematoxylin-eosin for morphological comparison of regenerative process with normal gland development seen by Fossati (2000)6. In order to check cell differentiation (here understood as capability to form/secrete cell products), we employed the histochemical technique of periodical reactive acid of Schiff (PAS), which is capable of evidencing formation of mucins and carbohydrates, which are components of primary salivary fluid 8-2.
MATERIAL AND METHODThe present study was submitted for analysis and approval by the Scientific Commission and Ethics Research Committee, Hospital de Clínicas de Porto Alegre, Project nº 01-048.
To complement the material used in the study, we analyzed slides representative of embryonic development of SMG in rats at 15, 16, 17, 18 and 19 days of fetal life. This material was integral part of the Doctorate studies by Fossati (2000)6.
Animals
We used in our study 20 Wistar male rats aged 30 (young) or 60 (adult) days, considered an excellent animal model to study SMG.
Surgical Technique
Animals were anesthetized with drugs Vetanarcol and Rompum in the proportion 1:1 and at 0.2ml dose for each 100g of body weigh, applied intramuscularly.
We performed trichotomy of the middle neck region, SMG was exposed and gland lobes were measured using drawing compass and millimeter rule.
The inferior third of the left lobe was removed, avoiding damage to any other structures. After suturing, animals were taken to the central animal lab, where they had ad libitum feed and water during the recovery period defined in the study 2, 3, 7 and 15 days postoperative.
They were divided based on age and duration of recovery, when we removed the glandular material. Both lobes were measured again, removed and immediately placed in fixating solution of Methacarn during 3 hours at room temperature.
Histological procedures
After fixation, we started routine histology processing, being that each piece was included in paraffin. We made 5?m sections using rotation microtome, being that some specimens were submitted to staining with hematoxylin-eosin and others to histochemical technique with periodical reactive acid of Schiff.
Observational Analysis
Histological pieces of the groups of young and adult animals in different regeneration periods were morphologically analyzed under hematoxylin-eosin staining.
Observational qualitative analysis of the obtained material was made by three observers under light microscope brand Olimpus model V-MDOB3, with magnification of 100x and 400x.
RESULTSMorphology
Regenerative process occurred in 100% of the analyzed cases and was quite early. After two days postoperative, young animals had surgical site with large amount of stroma, hemorrhagic areas, presence of inflammatory cells, wide angiogenesis and proliferation of rows of massive epithelial tissue (Figure 1A).
Three days postoperatively, we observed presence of lumen in some of the sites with probable apoptosis of central cells of these epithelial collections, and the abundant presence of stroma and inflammatory process cell elements (Figure 1B). This situation was modified seven days later, in which we detected reduction of connective tissue, owing to proliferation of epithelial tissue in search for a ramified pattern of SMG. This aspect was similar to that observed during the normal glandular development (Figures 1C and 2). In some specimens, at this time, we detected marked proliferation in structures similar to ducts, presenting central lumen, but still little differentiation (Figure 1D).
After 15 days, the regenerative process was similar to the remaining portion of SMG, presenting small amount of stroma, connective septa that formed lobules and epithelial structure of ducts and very complex secretory structure (data not shown).
In adult animals, the regenerative process proved to be delayed in comparison to young animals, with more rudimentary gland structures (Figure 3).
Cell Differentiation
Two days after the surgery, we observed rudiments with discreet staining of PAS, which was observed in the stroma and basal lamina of vessels (Figure 4A). Three days later, we could evidence cytoplasm of ducts and secretory endings in development (Figure 4B).
We detected decrease in the next stages, with few positive granules in the referred structures, marking was restricted to striated ducts at 15 days of regeneration (Figures 4C and 4D). The evaluation of normal development of SMG showed neutral mucins were initially marked at 17 days of fetal life in embryonic gland rudiments.
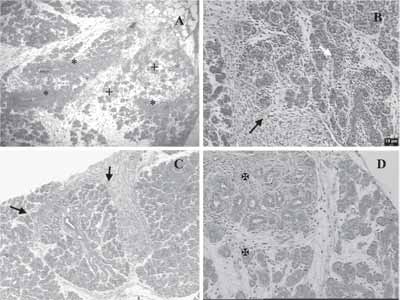
Figure 1
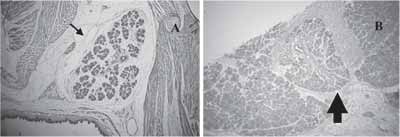
Figure 2
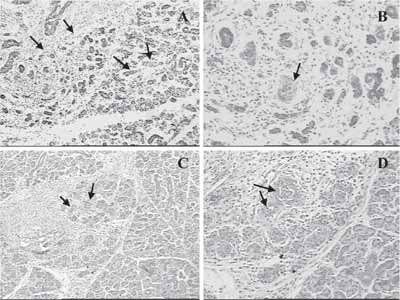
Figure 3
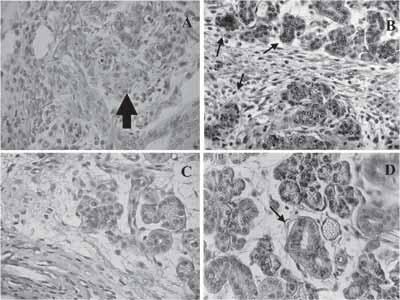
Figure 4
Similar results to the ones found by us have been reported by other authors 13-17. Aspects similar to normal development of SMG were observed in all stages, a fact that clearly differentiates the aspect found from simple organ regeneration. The region in process of regeneration was characteristic in all animals, regardless of age range. It was more advanced in young animals (30 days) in which glandular rudiments were better defined, showing more advanced development level. It can be explained by the fact that rats were in puberty, when granulous hormone-dependent duct becomes more prominent in male animals 18. The referred ductal segment, which is located between the intercalar and the striated duct, is a source of growth factors such as EGF (epidermal growth factor) and FGF (fibroblast growth factor), among others, which probably influences regeneration 19, 20. The area proved to be rich in connective tissue, abundant and highly cellularized stroma, especially in the first postoperative days. The population of inflammatory cells reduced its progression to regeneration, as well as ambient stroma. It seems to have as an important role in regeneration as in development 6, 21.
Hanks & Chaudhry (1971)13, upon studying regeneration of SMG in rats after partial excision, observed central necrotic region in the excised lobe on the 1st postoperative day with hemorrhagic areas, such as the ones visualized in this study still on the 2nd day of regeneration, in which recently-formed rudiments were few and sparse.
Similarly, in subsequent days we detected marked angiogenesis and large number of inflammatory cells that were present since the first day of observation. It is believed that essential nutrients to the process and inflammation-mediated substances (cytokines) that were installed performed an important role.
In many cases, especially three days after surgery, we detected marked proliferation of epithelial cells, similar to massive rows, presenting central lumen in some directions. These areas were originated from the remaining gland and directed to the process portion in formation. These findings were also reported by Hanks & Chaudhry (1971)13, who attributed them to cells of striated ducts remaining from surgery, which would originate epithelial cells in regeneration.
However, the authors of the present study disagreed since we believe that ducts are very undifferentiated to admit any characterization and even so, owing to the aspect of cells, position and nuclear size, they were much more similar to ducts of intercalar segment, which are known to have stem cells in their structure 22.
Takahashi & Wakita (1993)14 in their studies about regeneration of SMG in rats, reported that after gland ablation with laser radiation they identified similar structures to ducts, continuous with remaining ducts, which were regenerated in the periphery of the damaged lobe. In our study, the formation of new secretory pieces seemed to happen from the remaining structures, with subsequent growth depending on differentiation and progressive proliferation of recently formed cells. The statement that it would stem from the ducts, as reported by the literature, does not seem to be common to all analyzed cases.
Gland regeneration that is derived from partial excision of the organ, or after atrophy that is followed by ligation to the excreting duct, is processed in many different ways. This fact is well understood and it is due to the nature and extension of the damage to the gland, and to blood supply to the involved region. Takahashi et al. (1998)23 noticed in their study that in regeneration that occurred after parotid gland atrophy caused by ligation of the excreting duct, only ductal cells were seen in the atrophic region, without presence of any acinar cell. This fact was attributed to probable apoptosis of these cells, being that this aspect was observed in our study in some specimens.
However, this fact was not confirmed by Colombo (2001)24, who conducted obstruction of parotid gland and did not find enough number of ductal cells PCNA-positive after removal of ligation.
Ihler et al. (2002)25, working with human salivary glands, both normal and metaplastic, noticed that in normal state, luminal cells of acinar and intercalar ducts regenerate regardless of basal or myoepithelial cell proliferation, whereas in striated and terminal ducts regeneration of cells depend on proliferation of basal cells.
A similar aspect of SMG in rats at 19 days of fetal life was found after seven days of surgery, with more differentiated ducts and secretory extremities, even in little developed parenchyma, surrounded by abundant stroma. Many apoptotic figures were seen at this time, both in ducts and future acinar region, in the central portion. This fact was also observed by Hayashi et al. (2000)26, who used Tunel technique, light and electron microscopy, and found apoptotic cells that would be involved in reduction of number of cells of terminal tubule, and formation of gland lumen, in post-natal development of rats' SMG.
After 15 days of regeneration it was observed a similar aspect to adult gland, involved by a fibrous capsule. It was observed by Hanks & Chaudhry (1971)13, who detected that these cells in differentiation were organized in lobules three weeks after surgery. Takahashi et al. (1998)23, however, observed this situation even earlier, after 10 days of regeneration, which was followed by ductal obstruction of parotid gland. This difference in times of total glandular regeneration is owed to greater destruction of the organ that occurs in partial ablation. Contrarily to what was observed by the first authors, regeneration observed when part of the gland was lost was more advanced in the proximal region than the non-damaged portion of the gland, being more delayed in the periphery of the organ (data not shown).
In our study we used histochemical technique with PAS in order to define the onset of neutral mucin production by regenerated SMG. We could observe that production of neutral mucin (a fact that reveals cell differentiation) was already present, in a discreet form, two days after regeneration, showing precocity of cytodifferentiation. Marking was intensified after three days, with strong marking of cytoplasm of duct cells and secretory cells, being characterized as the peak of mucin production. In the following stages there was decrease, with few positive granules in the mentioned structures, restricted to walls of striated ducts, 15 days after the surgery, a similar situation to that found in intact glands.
Comparing with fetal gland material, we could observe that the marking peak was on the 17th day of gestation, therefore, with more mature development, since it would correspond in our study to the 5th day of regeneration.
Regeneration process of excised SMG in rats is stable, permanent and gradual, following the defined stages.
CONCLUSIONWe could confirm in our study that gland regeneration that followed partial ablation started very early, probably right after excision, and it was more advanced in animals in puberty, and it occurred thanks to the influence of components of the abundant extracellular matrix. Cytodifferentiation seems to be earlier than during normal gland development and it changes location with process progression.
REFERENCES1. Kagamy H, Hiramatsu Y, Hishida S, Okazaki Y, Horie K, Oda Y, Ueda M. Salivary growth factors in health and disease. Adv Dent Res 2000; 14:99-102.
2. Nagy, G. Role of saliva, salivary glands and Epidermal Growth Factor (EGF) on oral wound healing. Fogorv Sz 2003; 96(1):17-20.
3. Thylstrup A, Fejerskov O. Cariologia Clínica. 2a Edição São Paulo: Santos; 1995. 13-6.
4. Cutler L, Chaudry A. Intercellular contacts at the epithelial-mesenchymal interface during the prenatal development of the rat submandibular gland. Dev Biol 1973; 33:229-40.
5. Cutler L, Chaudry A. Cytodifferentiation of the acinar cells of the rat submandibular gland. Dev Biol 1974; 41:31-41.
6. Fossati ACM. Integrinas e proteínas de matriz extracelular durante o desenvolvimento da glândula submandibular de ratos. São Paulo [Tese De Doutorado]. Instituto De Ciências Biomédicas, USP, 2000, 62f.
7. Spooner BS, Hardman P. Localization of extracellular matrix components in developing mouse salivary glands by confocal microscopy. Anat Rec 1992; 234:452-9.
8. Gal R, Koren R, Nofech-Mozes S, Mukamel E, His Y, Zajdel L. Evaluation of mucinous metaplasia of the prostate gland by mucin histochemistry. Br J Urol 1996; 77(1):113-7.
9. Harrison JD, Auger DW, Paterson KL, Rowley PS. Mucin histochemistry of submandibular and parotid salivary glands of man: light and electron microscopy. Histochem J 1997; 19(10-11):555-64.
10. Kodaira H, Ishihara K, Hotta K, Kagoshima M, Shimada H, Ishii K. Rat gastric mucous gel layer contains sialomucin not produced by the stomach. Jpn J Pharmacol 1999; 81(1):86-93.
11. Liman N, Bayram G, Kocak M. Histological and histochemical studies on the lingual, preglottal and laryngeal salivary glands of the Japanese quail (Coturnix Coturnix Japonica) at the post-hatching period. Anat Histol Embryol 2001; 30(6):367-73.
12. Slomiany BL, Murty VL, Slomiany A. Structural features of carbohydrate chains in human salivary mucins. Int J Biochem 1993; 25(2):259-65.
13. Hanks CT, Chaudhry AP. Regeneration of rat submandibular gland following partial extirpation - a light and electron microscopic study. Am J Anat 1971; 130(2):195-207.
14. Takahashi S, Wakita M. Regeneration of the intralobular duct and acinus in rat submandibular glands after yag laser irradiation. Arch Histol Cytol 1993; 56(2):199-206.
15. Takahashi S, Wakita M. Cytokeratin expression during regeneration of the intralobular duct in rat submandibular glands after YAG laser irradiation. Arch Histol Cytol 1994; 57(2):167-73.
16. Takahashi S, Domon T, Yamamoto T, Wakita M. Regeneration of myoepithelial cells in rat submandibular glands after yttrium aluminum Garnett Laser Irradiation. Int J Exp Pathol 1997; 78(2):91-9.
17. Takahashi S, Nakamura S, Suzuki R, Domon T, Yamamoto T, Wakita M. Changing myoepithelial cell distribution during regeneration of rat parotid glands. Int J Exp Pathol 1999; 80(5):283-90.
18. Garcia HF, Garcia-Poblete E, Moro-Rodriguez E, Catala-Rodriguez M, Rico-Morales ML, Garcia-Gomez De Las Heras MS. Histomorphometrical study of the submandibular gland ductal system in the rat. Histol Histopathol 2002; 17(3):813-6.
19. Amano O, Iseki S. Expression and localization of cell growth factors in the salivary gland: a review. Kaibogaku Zasshi 2001; 76(2):201-12.
20. Okazaki Y, Kagami H, Hattori T, Hishida S, Shigetomi T, Ueda M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol 2000; 45(10):911-9.
21. Kashimata M, Gresik EW. Epidermal growth factor system is a physiological regulator of the development of the mouse fetal submandibular gland and regulates the expression of the 6-integrin subunit. Dev Dyn 1997; 208:149-61.
22. Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec 2001; 263(2):202-14.
23. Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol 1998; 79(5):293-301.
24. Colombo CED. Atrofia e regeneração da glândula parótida após ligadura do ducto excretor: estudo histológico, histoquímico e imuno-histoquímico em ratos. São José Dos Campos [Dissertação De Mestrado]. Faculdade De Odontologia, Universidade Estadual Paulista, 2001, 130f.
25. Ihrler, S.; Zietz, C.; Sendelhofert, A.; Lang, S.; Blasenbreu-Vogt, S.; Lohrs, U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch 2002; 440(5): 519-26.
26. Hayashi H, Ozono S, Watanabe K, Nagatsu I, Onozuka M. Morphological aspects of the postnatal development of submandibular glands in male rats: involvement of apoptosis. J Histochem Cytochem 2000; 48(5):695-8.
1 Ph.D. in Cell and Tissue Biology, USP, Master in Dental Sciences, Odontopediatrics - USP, Joint Professor, Mouth and Dental History and Embryology - Department of Morphological Sciences, Institute of Healthcare Basic Sciences, Federal University of Rio Grande do Sul (UFRGS).
2 Undergraduates, Dental School, UFRGS.
Institute of Healthcare Basic Sciences- School of Dental Sciences - Discipline of Mouth and Dental Histology and Embryology - Laboratory of Mouth Biology - UFRGS.
Address correspondence to: Prof Dra. Anna C. M. Fossati - R. Ramiro Barcelos, 2492 5º andar sala 503 Porto Alegre RS 90035-003
Tel (55 51) 33165024 - Fax (55 51) 3316-5439 - E-mail: annafo@ufrgs.br


