INTRODUCTIONRadiotherapy is one of the preferred treatment options to patients who have head and neck neoplasm even though we can see that sequelae are inevitable. Such sequelae include dermatitis in the radiated regions, radiation dental decays, especially in poor condition mouths, xerostomia if radiation reaches major salivary glands, and inflammatory or infectious abnormalities of the oral cavity, generically named mucositis.
Abreu & Silva2 stated that mucosa affections after radiotherapy develop with doses greater than 3,000 cGy causing discomfort and dysphagia and leading to nutritional impairment. Mucositis is normally transient and patients recover spontaneously within the first month after the completion of treatment. Among the manifestations of mucositis, ulceration is the most important one, being an open door for bacterial infections and determining in some cases interruption of radiotherapy treatment. The association between radiotherapy and chemotherapy agents produces a synergic effect maximizing severity of oral mucosa inflammatory complications.
Despite the several studies published, there is no consensus concerning treatment or prevention of mucositis. Many substances and drugs have been studied, but no one was confirmed as being the best method to solve the problem. Gluconate chlorhexidine is a widely used drug in dental sciences, especially in the areas of periodontics, surgery and implants, considering that its main characteristic is to fight against the bacterial plaque.
The purpose of the present study was to assess the effects of 0.12% gluconate chlorhexidine on oral mucosa secondary reactions and quality of life reported by patients with head and neck cancer submitted to radiotherapy.
MATERIAL AND METHODThe present study was a prospective, double blind placebo-controlled study conducted at Institute of Radiotherapy of São Paulo, Casa de Saúde Santa Rita, in São Paulo, between May and October 2000, including 30 patients with malignant head and neck neoplasm with exclusive indication of radiotherapy. Patients were indicated to such management modality because cancer was advanced, it was impossible to treat them surgically or because of medical option, and they were divided into two groups of 15 subjects each, according to the use of medication or placebo.
In the development of the study, 9 patients were excluded owing to lack of assessment or interruption of treatment without medical prescription - 4 in the placebo group and 5 in the medication group.
The medication (gluconate chlorhexidine at 0.12%) was used without dilution and all patients were instructed to conduct two mouthwashes twice a day with the substance given by the examiner, for 1 minute, and with a 12-hour interval between sessions. Both the medication and the placebo vials were distributed randomly by an assistant of the researcher, who was the only person who knew which substance was given to whom.
Weekly assessment included oral clinical examination to check mucosa abnormalities and a questionnaire to check quality of life perception.
Mucosa affections, observed in the clinical examination, were classified according to the criteria defined by the World Health Organization and the Group of Therapy for Radiation in Oncology, described by Trotti et al.3. In summary, they classified as grade 0 absence of signs of mucositis, grade I as the presence of erythema, grade II as the presence of pseudomembranes smaller than 1.5 cm in diameter, grade III as the presence of pseudomembranes larger than 1.5 cm in diameter, and grade IV as the presence of ulceration.
The assessed aspects in the questionnaire about quality of life perception were presence of pain (numerical scale from 0 to 4, being 0 total absence of pain and 4 intense pain), loss of taste, difficulty to swallow caused by pain or other causes, and change in eating habits related to the change in consistency of foods, replacing some foods or modifying the way they were prepared.
The characteristics of age, gender, diagnosis, use of medication or placebo, total radiated dose, pain, appetite abnormalities, modification of eating habits and taste before radiotherapy are demonstrated in Table 1.
Results were analyzed by Fisher's Exact test with statistic significance level of 95% (p<0.05).
RESULTSSymptoms related to the referred quality of life
Table 2 shows the distribution of frequency of symptoms related to quality of life referred by patients and based on the questionnaire. None of the assessed characteristics present any permanent differences during the radiotherapy procedure.
All patients in the placebo group presented pain at some time in the evolution and on the 4th week of radiotherapy there was a statistically significant difference between group frequency. The intensity of the pain followed the same pattern, being more intense only on the 4th week.
Owing to the results obtained, we could notice that all patients reported eating habits changes without any perceptible differences between the groups. Appetite abnormalities were more constant in the placebo group and there was statistically significant difference on the 3rd week of treatment.
Data showed that on the first week of radiotherapy there was a significant increase in number of patients that used chlorhexidine and related taste changes, which could suggest that a side effect of the drug to this patients was anticipated loss of gustatory sensation. By the end of the study, there was a trend of total loss of taste in all patients.
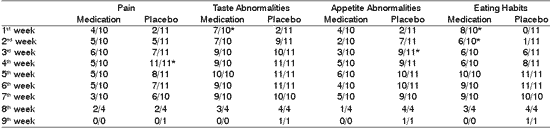
Clinical signs of the oral examinations during radiotherapy
Table 3 shows the distribution of frequencies of abnormalities detected by the oral examination in different periods of treatment. Data demonstrated that at the end of the second week all patients that did not use chlorhexidine presented erythema of oral mucosa, differently from the group with medication, in which less than half had the same abnormality. In subsequent assessments, we detected a trend towards no differences between the two groups, with all patients developing this manifestation of mucositis.
The presence of pseudomembranes was more frequent in the placebo group on the 3rd to 6th week of treatment and we did not find statistically significant difference comparing to the clinical evidence of formation of oral mucosa ulceration, which was low in both studied groups.
Table 4 shows the grading of mucositis in patients in both studied groups. We can notice a trend of higher grades in patients of the placebo group after the 3rd to 8th week of radiotherapy.
Table 1. Distribution of age, gender, total dose of radiotherapy and clinical characteristics previous to radiotherapy (presence of pain, appetite abnormalities, taste and eating habits).
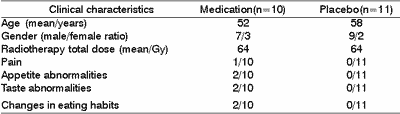
Table 2. Distribution of frequency of characteristics of quality of life referred by patients after the beginning of radiotherapy.
* statistically significant differences (p<0.05)
Table 3. Distribution of frequency of the clinical characteristics of oral examinations in patients after the beginning of radiotherapy.
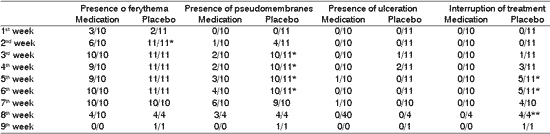
* statistically significant differences (p<0.05)
Table 4. Distribution of mucositis grading frequency (according to the Group of Therapy for Radiation in Oncology 3) in both groups of patients after the beginning of therapy.
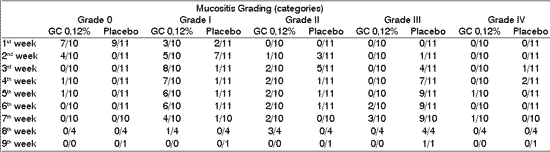
* statistically significant differences (p<0.05)
Oral mucositis is one the most frequent complications in patients with head and neck cancer submitted to radiotherapy treatment 4, 5.
Among the various factors implied in the genesis of mucosa abnormalities induced by radiation, we can include the modification of the bacterial oral flora with development of infectious episodes. Thus, an antiseptic medication could help preventing such modifications and reduce the intensity of mucositis episodes. The medication chosen was gluconate chlorhexidine at 0.12%, a widely used drug in the area of dental sciences 6, 7.
The treatment of mucositis should be conservative to avoid accentuation of tissue irritation and damage to the remaining cells of the epithelium followed by control of the bacterial plaque and maintenance of oral hygiene 8.
Mucosa abnormalities are complicating factors that sometimes cause interruption of the radiotherapy treatment, hindering the final result of treatment 9, 10. This fact is characterized in the data of the present study since many subjects evolved to treatment interruption, but less frequently in patients that used chlorhexidine on the 6th and 8th weeks.
Not always interruption of treatment is the most harmful effect of post-radiotherapy mucositis. In some patients, the mucosa becomes excessively painful, hindering swallowing, a fact that impairs the nutritional status of the patients 11, already deteriorated by the underlying disease. All studied patients had pain, more or less intense, throughout the radiotherapy treatment and it was not a factor that differentiated it from other groups. Taste abnormalities, owing to gustatory papillae affection by radiation 12, can influence food intake and contribute to worsening of nutritional status and quality of life of patients.
The results presented here showed that even though chlorhexidine had not ceased pain in patients, it was less frequent in the placebo group, leading to the conclusion that there is a tendency in chlorhexidine to alleviate painful symptoms during radiotherapy.
Both groups of patients presented pain throughout treatment but less intensity in subjects that used chlorhexidine showing better quality of life in this group. Since there is no direct analgesic effect in the medication, we can deduct that differences among other parameters (low grade of erythema and pseudomembrane) could have contributed to such fact.
Since patients with mucositis have difficulty in swallowing and ingesting at some time in treatment, the form of application of the selected medication (topical by mouthwash) proved to be reasonable, since even during impaired intake periods, subjects do not have to interrupt treatment. In addition, mouthwash with chlorhexidine also provides mechanical cleaning of the mouth acting in necrotic tissues 13.
In some cases, mucositis leads to formation of ulcers and when it happens, there is the possibility of secondary and opportunistic infections, especially by Candida albicans14. For this reason, the selected drug should have antimicrobial action, a fact that is well known as the action of chlorhexidine 11-13, 15.
Similarly to the abnormalities induced by chemotherapy, ulcerative lesions of the mouth and oropharynx after radiotherapy develop 7 to 14 days after the beginning of treatment 16. Al-Tikriti et al.17 assessed patients with oral cancer with direct radiation and noticed that after one week patients had already presented reduction of salivary flow, some erythema and pain plus worsening of severity of the abnormalities after the third week.
Many authors 7, 18, 19 reinforced the need for perfect oral hygiene during radiotherapy and showed that the groups with the highest level of oral hygiene had less intense mucositis. Our data indicated that the use of chlorhexidine led to less severe grades of mucositis.
Generally speaking, we noticed that all patients, at a specific time, were forced to change their eating habits. As to taste, there was a trend for patients with chlorhexidine to present more frequent abnormalities, suggesting that a side effect of the drug is the loss of gustatory sensation. It is worth mentioning that all patients presented loss of tastes, in accordance with other authors who also stated that radiation causes loss of gustatory papillae and, consequently, loss of taste 9, 20. Our data of taste abnormality exceeded the estimates of Epstein et al. who published a frequency of 75.4% of patients affected by taste abnormalities 21.
In the clinical assessment of patients, we tried to observe the main abnormalities associated with mucositis, being one of them the presence of erythema. Oral mucosa erythema is an important clinical sign because it is one of the main signs of mucosa inflammation and is detected approximately after 2,500 cGy. In this stage, the patient starts to have difficulty to swallow and masticate solid foods 20. The fact that the drug is not anti-inflammatory and presents a protective effect on onset of erythema reinforced the hypothesis that the initial inflammation is related to the presence of bacterial plaque.
Some subjects in our study presented mucosa erythema as of the first week of treatment, that is, with doses lower than 2,500 cGy. Despite early, such findings are in accordance with the studies by Al-Tikriti et al. 17, which published indicative data that after one week, patients submitted to radiotherapy had already manifested some erythema.
The results of presence of oral mucosa ulceration did not differentiate both groups and did not provide enough data to conclude whether the drug was beneficial or protective in this aspect.
CONCLUSIONFoote et al. 22, in a study of the assessment of chlorhexidine efficacy in radiotherapy-induced mucositis, concluded that differently from what was initially assumed, this drug would not be indicated owing to the discomfort and taste abnormalities that followed it use. Our findings did not confirm this statement about chlorhexidine; despite the fact that it did not prevent the occurrence of radiotherapy-induced mucositis, it caused reduction of the severity of the clinical picture, as users of the medication presented less severe pictures and lower grades of mucositis.
REFERENCES 1. Symonds RP, Mcilroy P, Khorrami J, Paul J, Pyper E, Alcock SR et al. The reduction of radiation by selective decontamination antibiotic pastilles: a placebo-controlled double-blind trial. Br J Cancer 1996;74:312-7.
2. Abreu CEV, Silva JLF. Teleterapia. In: Parise Jr O. Câncer de Boca - Aspectos Básicos e Terapêuticos. São Paulo: Sarvier; 2000.
3. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, Mccormick B, Morris M, Rich T, Shipley WE, Curran W. Common Toxicity Criteria: version 2.0 an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:13-47.
4. Rosenthal LE, Wilkie B. The effects of radiotherapy on oral tissues. J Pros Den 1965;151:153-6.
5. Bernhoft CH, Skaug N. Oral findings in irradiated dentulous patients. Int J Oral Surg 1985;14:416-27.
6. Ciancio S. Expanded and future uses of mouth rinses. JADA 1994;125:29S-32S.
7. Logothetis D, Martinz-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. JADA 1995;126.
8. Borowski E, Benhamon E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation. A randomized controlled trial comparing two protocols of dental care. Oral Oncol Eur J Cancer 1994;30B:93-7.
9. Mchelroy H, Guerra O. Oral management of the head and neck radiation patient. Mo Med 1985;81:15-8.
10. Freire RCCG, Kowalski LP, Dib LL, Ribeiro KCB. Risk factors for severe oral mucositis induced by radiotherapy. Proceedings of the 1st World Congress on Head and Neck Oncology. 1998. Bologna. Italy: Monduzzi Editore.
11. Kolbinson DA, Schubert MM, Flournoy N, Truelove EL. Early oral changes following bone marrow transplantation. Oral Surg Oral Med Oral Pathol 1988;66:130-8.
12. Petitto JV. Complicações e seqüelas da radioterapia nos cânceres da cavidade oral. In: Brandão LG, Ferraz AR. Cirurgia de Cabeça e Pescoço. São Paulo: Rocca; 1989.
13. Spijkervet FKL, Van Saene HKF, Van Saene JJM, Panders AK, Vermey A, Mehta DM et al. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck. J Surg Oncol 1991;46:167-73.
14. Pinto DS, Dib LL. Papel do cirurgião dentista no diagnóstico do câncer de cabeça e pescoço. In: Brandão LG, Ferraz AR. Cirurgia de Cabeça e Pescoço. São Paulo: Rocca; 1989.
15. Barros LM, Fiorini JE. Efeito da clorexidina e da água ozonizada sobre o S. viridans da placa bacteriana supragengival. Rev APCD 2000;54:47-52.
16. Bruya MA, Madeira NP. Stomatitis after chemotherapy. Am J Nurs 1975;75:1349-52.
17. Al-Tikriti U, Martin MV, Bramley PA. A pilot study of the clinical effects of irradiation on the oral tissues. Br J Oral Max Surg 1974;22:77-86.
18. Keene HJ, Daly T, Brown LR, Dreizen S, Drane JB, Horton IM et al. Dental caries and Streptococcus mutans prevalence in cancer patients with irradiation-induced xerostomia 1-13 years after radiotherapy. Caries Res 1981;15:416-27.
19. Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dentist 1998;18:189-93.
20. Dib LL, Curi MM. Complicações Orais na Oncologia. In: Salvajoli JV, Souhami L, Faria SL. Radioterapia em Oncologia. Rio de Janeiro: Medsi; 1999.
21. Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson-Moore P et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck 1999;21:1-11.
22. Foote RL, Loprinzi CL, Frank AR, O'fallon J, Gulavita S, Tewfik HH et al. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol 1994;12:2630-3.
1 Master in Head and Neck Surgery - Post-Graduation Course, Hospital Heliópolis.
2 Head of the Head and Neck Surgery Unit, Hospital Heliópolis, Master in Sciences, Post-Graduation Course, Hospital Heliópolis.
3 Professor, Post-Graduation Course on Head and Neck Surgery, Hospital Heliópolis, Ph.D. in Endocrinology, UNIFESP-EPM.
Study conducted at the Head and Neck Surgery Unit, Hospital Heliópolis, São Paulo.
Address correspondence to: Odilon Victor Porto Denardin - Curso de Pós- Graduação em Cirurgia de Cabeça e Pescoço/ Hospital Heliópolis -
Rua Cônego Xavier, 276 10º andar Sacomã 04231-030 São Paulo
Tel (55 11) 6215-2837/ 273-8224/ 9259-7331
Article submitted on March 11, 2003. Article accepted on May 13, 2003.


